Synergistic dopaminergic neurotoxicity of manganese and lipopolysaccharide: differential involvement of microglia and astroglia
- PMID: 19895668
- PMCID: PMC2809141
- DOI: 10.1111/j.1471-4159.2009.06477.x
Synergistic dopaminergic neurotoxicity of manganese and lipopolysaccharide: differential involvement of microglia and astroglia
Abstract
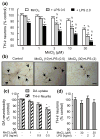
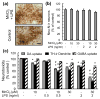
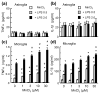
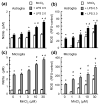
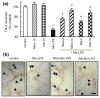
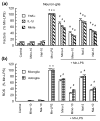
Overexposure to manganese is known to cause damage to basal ganglial neurons and the development of movement abnormalities. Activation of microglia and astrocytes has increasingly been associated with the pathogenesis of a variety of neurological disorders. We have recently shown that microglial activation facilitates manganese chloride (MnCl2, 10-300 microM)-induced preferential degeneration of dopamine (DA) neurons. In this study, we report that combinations of MnCl2 (1-30 microM) and endotoxin lipopolysaccharide (LPS, 0.5-2 ng/mL), at minimally effective concentrations when used alone, induced synergistic and preferential damage to DA neurons in rat primary neuron-glia cultures. Mechanistically, MnCl2 significantly potentiated LPS-induced release of tumor necrosis factor-alpha and interleukin-1 beta in microglia, but not in astroglia. MnCl2 and LPS were more effective in inducing the formation of reactive oxygen species and nitric oxide in microglia than in astroglia. Furthermore, MnCl2 and LPS-induced free radical generation, cytokine release, and DA neurotoxicity was significantly attenuated by pre-treatment with potential anti-inflammatory agents minocycline and naloxone. These results demonstrate that the combination of manganese overexposure and neuroinflammation is preferentially deleterious to DA neurons. Moreover, these findings not only shed light on the understanding of manganese neurotoxicity but may also bear relevance to the potentially multifactorial etiology of Parkinson's disease.
Figures


References
-
- Bae JH, Jang BC, Suh SI, Ha E, Baik HH, Kim SS, Lee MY, Shin DH. Manganese induces inducible nitric oxide synthase (iNOS) expression via activation of both MAP kinase and PI3K/Akt pathways in BV2 microglial cells. Neuroscience letters. 2006;398:151–154. - PubMed
-
- Barhoumi R, Faske J, Liu X, Tjalkens RB. Manganese potentiates lipopolysaccharide-induced expression of NOS2 in C6 glioma cells through mitochondrial-dependent activation of nuclear factor kappaB. Brain Res Mol Brain Res. 2004;122:167–179. - PubMed
-
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44:1583–1586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

