The proline-rich domain of tau plays a role in interactions with actin
- PMID: 19895707
- PMCID: PMC2784441
- DOI: 10.1186/1471-2121-10-81
The proline-rich domain of tau plays a role in interactions with actin
Abstract
Background: The microtubule-associated protein tau is able to interact with actin and serves as a cross-linker between the microtubule and actin networks. The microtubule-binding domain of tau is known to be involved in its interaction with actin. Here, we address the question of whether the other domains of tau also interact with actin.
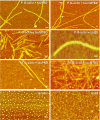
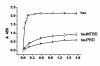
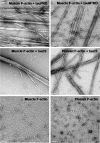
Results: Several tau truncation and deletion mutants were constructed, namely N-terminal region (tauN), proline-rich domain (tauPRD), microtubule binding domain (tauMTBD) and C-terminal region (tauC) truncation mutants, and microtubule binding domain (tauDeltaMTBD) and proline-rich domain/microtubule binding domain (tauDeltaPRD&MTBD) deletion mutants. The proline-rich domain truncation mutant (tauPRD) and the microtubule binding domain deletion mutant (tauDeltaMTBD) promoted the formation of actin filaments. However, actin assembly was not observed in the presence of the N-terminal and C-terminal truncation mutants. These results indicate that the proline-rich domain is involved in the association of tau with G-actin. Furthermore, results from co-sedimentation, solid phase assays and electron microscopy showed that the proline-rich domain is also capable of binding to F-actin and inducing F-actin bundles. Using solid phase assays to analyze apparent dissociation constants for the binding of tau and its mutants to F-actin resulted in a sequence of affinity for F-actin: tau >> microtubule binding domain > proline-rich domain. Moreover, we observed that the proline-rich domain was able to associate with and bundle F-actin at physiological ionic strength.
Conclusion: The proline-rich domain is a functional structure playing a role in the association of tau with actin. This suggests that the proline-rich domain and the microtubule-binding domain of tau are both involved in binding to and bundling F-actin.
Figures









References
-
- Brandt R. The tau proteins in neuronal growth and development. Front Biosci. 1996;1:d118–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

