Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a
- PMID: 19895849
- PMCID: PMC2815128
- DOI: 10.1016/j.imlet.2009.10.007
Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a
Abstract
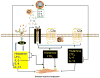
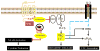
Allergic diseases such as asthma result from inappropriate immunologic responses to common environmental allergens in genetically susceptible individuals. Following allergen exposure, interaction of dendritic cells (DC) with CD4(+) T cells leads to the production of Th2 cytokines, which induce B cells to synthesize IgE molecules (sensitization phase). These IgE molecules bind to their high affinity receptors (FcvarepsilonRI) on the surface of mast cells and basophils and their subsequent cross-linking by allergen results in the release of preformed and newly synthesized mediators, which cause bronchoconstriction, lung inflammation and airway hyperresponsiveness (AHR) in asthma (effector phase). The complement components C3a and C5a levels are increased in the lungs of patients with asthma and are likely generated via the actions of both allergen and mast cell proteases. In vivo studies with rodents have shown that while C3a facilitates allergen sensitization in some models C5a inhibits this response. Despite this difference, both anaphylatoxins promote lung inflammation and AHR in vivo indicating that cells other than DC and T cells likely mediate the functional effects of C3a and C5a in asthma. This review focuses on the contribution of C3a and C5a in the pathogenesis of asthma with a particular emphasis on mast cells and basophils. It discusses the mechanisms by which anaphylatoxins activate mast cells and basophils and the associated signaling pathways via which their receptors are regulated by priming and desensitization.
Figures

References
-
- Beaven MA, Hundley TR. Mast cell related diseases: genetics, signaling pathways, and novel therapies. In: Finkel TH, Gutkind JS, editors. Signal Transduction and Human Disease. Hoboken, NJ: 2003. pp. 307–355.
-
- US CDC. Forecasted state-specific estimates of self reported asthma prevalence-United States. MMWR Morb Mortal Wkly Rep. 1998;47:1022–1025. - PubMed
-
- Tarantini F, Baiardini I, Passalacqua G, Braido F, Canonica GW. Asthma treatment: ‘magic bullets which seek their own targets’. Allergy. 2007;62:605–610. - PubMed
-
- Kuhn R. Immunoglobulin E blockade in the treatment of asthma. Pharmacotherapy. 2007;27:1412–1424. - PubMed
-
- Djukanovic R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170:583–593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

