Universal influenza DNA vaccine encoding conserved CD4+ T cell epitopes protects against lethal viral challenge in HLA-DR transgenic mice
- PMID: 19895924
- PMCID: PMC3364000
- DOI: 10.1016/j.vaccine.2009.10.103
Universal influenza DNA vaccine encoding conserved CD4+ T cell epitopes protects against lethal viral challenge in HLA-DR transgenic mice
Abstract
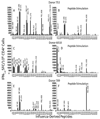
The goal of the present study was to design a vaccine that would provide universal protection against infection of humans with diverse influenza A viruses. Accordingly, protein sequences from influenza A virus strains currently in circulation (H1N1, H3N2), agents of past pandemics (H1N1, H2N2, H3N2) and zoonotic infections of man (H1N1, H5N1, H7N2, H7N3, H7N7, H9N2) were evaluated for the presence of amino acid sequences, motifs, that are predicted to mediate peptide epitope binding with high affinity to the most frequent HLA-DR allelic products. Peptides conserved among diverse influenza strains were then synthesized, evaluated for binding to purified HLA-DR molecules and for their capacity to induce influenza-specific immune recall responses using human donor peripheral blood mononuclear cells (PBMC). Accordingly, 20 epitopes were selected for further investigation based on their conservancy among diverse influenza strains, predicted population coverage in diverse ethnic groups and capacity to recall influenza-specific responses. A DNA plasmid encoding the epitopes was constructed using amino acid spacers between epitopes to promote optimum processing and presentation. Immunogenicity of the DNA vaccine was measured using HLA-DR4 transgenic mice and the TriGrid in vivo electroporation device. Vaccination resulted in peptide-specific immune responses, augmented HA-specific antibody responses and protection of HLA-DR4 transgenic mice from lethal PR8 influenza virus challenge. These studies demonstrate the utility of this vaccine format and the contribution of CD4(+) T cell responses to protection against influenza infection.
Figures



References
-
- WHO. Influenza Fact Sheet No. 211. http://www.who.int/mediacentre/factsheets/fs211/en/.
-
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med. 2005;165:265–272. - PubMed
-
- Beveridge WI. Webster R, Bean W., Jr . Evolution and ecology of human influenza viruses. London: Heinemann; 1977. Influenza: the last great plague.
-
- Oxford JS. Influenza A pandemics of the 20th century. Rev Med Virol. 2000;10:119–133. - PubMed
-
- Belser JA, Blixt O, Chen LM, Pappas C, Maines TR, Van Hoeven N, Donis R, Busch J, McBride R, Paulson JC, Katz JM, Tumpey TM. Contemporary North American influenza H7 viruses possess human receptor specificity: Implications for virus transmissibility. Proc Natl Acad Sci USA. 2008;105:7558–7563. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

