FoxO3 regulates neural stem cell homeostasis
- PMID: 19896443
- PMCID: PMC2775802
- DOI: 10.1016/j.stem.2009.09.014
FoxO3 regulates neural stem cell homeostasis
Abstract
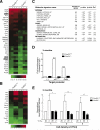
In the nervous system, neural stem cells (NSCs) are necessary for the generation of new neurons and for cognitive function. Here we show that FoxO3, a member of a transcription factor family known to extend lifespan in invertebrates, regulates the NSC pool. We find that adult FoxO3(-/-) mice have fewer NSCs in vivo than wild-type counterparts. NSCs isolated from adult FoxO3(-/-) mice have decreased self-renewal and an impaired ability to generate different neural lineages. Identification of the FoxO3-dependent gene expression profile in NSCs suggests that FoxO3 regulates the NSC pool by inducing a program of genes that preserves quiescence, prevents premature differentiation, and controls oxygen metabolism. The ability of FoxO3 to prevent the premature depletion of NSCs might have important implications for counteracting brain aging in long-lived species.
Figures
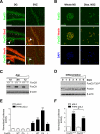
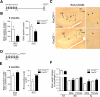

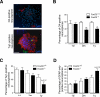
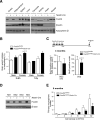
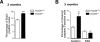
Comment in
-
Preview. The Tortoise, the hare, and the FoxO.Cell Stem Cell. 2009 Nov 6;5(5):451-2. doi: 10.1016/j.stem.2009.10.011. Cell Stem Cell. 2009. PMID: 19896431
References
-
- Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. - PubMed
-
- Alvarez-Buylla A, Temple S. Stem cells in the developing and adult nervous system. J Neurobiol. 1998;36:105–110. - PubMed
-
- Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell. 2007;28:941–953. - PubMed
-
- Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging. 2004;25:333–340. - PubMed
-
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

