FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis
- PMID: 19896444
- PMCID: PMC3285492
- DOI: 10.1016/j.stem.2009.09.013
FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis
Abstract
The PI3K-AKT-FoxO pathway is integral to lifespan regulation in lower organisms and essential for the stability of long-lived cells in mammals. Here, we report the impact of combined FoxO1, 3, and 4 deficiencies on mammalian brain physiology with a particular emphasis on the study of the neural stem/progenitor cell (NSC) pool. We show that the FoxO family plays a prominent role in NSC proliferation and renewal. FoxO-deficient mice show initial increased brain size and proliferation of neural progenitor cells during early postnatal life, followed by precocious significant decline in the NSC pool and accompanying neurogenesis in adult brains. Mechanistically, integrated transcriptomic, promoter, and functional analyses of FoxO-deficient NSC cultures identified direct gene targets with known links to the regulation of human brain size and the control of cellular proliferation, differentiation, and oxidative defense. Thus, the FoxO family coordinately regulates diverse genes and pathways to govern key aspects of NSC homeostasis in the mammalian brain.
Figures

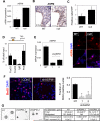
 ) pups. There are more S100 or NeuN-positive cells in FoxO null brain compared with the control (*, p<0.1). ** indicates the region where S100+ and NeuN+ cells were scored. hGFAP-cre mice were crossed with ROSA26R mice, where Cre-mediated recombination drives the constitutive expression of the ß-galactosidase. X-gal staining positice cells are shown in blue. (C) Increase in cell cycle re-entry of FoxO null SVZ cells. Young mice (P8) were single-pulse labeled with BrdU 24hrs prior to the sacrifice and brain sections were stained for BrdU (red), Ki67 (green), and Sox2 (cyan). The fraction of Sox2 positive cells re-entered cell cycle (open arrow, BrdU+/Ki67+/Sox2+ triple positive) increased in FoxO null SVZ and that of no longer dividing (filled arrow, BrdU+/Ki67-/Sox2+) is higher in WT SVZ. Percent mean ±s.d. of Ki67+ cells from BrdU+/Sox2+ cells from 10 WT and 6 FoxO null mice is shown ( *, p<0.001 by two tail t-test). Bar=40μm (D) Ki67 positive NSC of FoxO WT and null cultures (n=5, P2). Bar=20μm.
) pups. There are more S100 or NeuN-positive cells in FoxO null brain compared with the control (*, p<0.1). ** indicates the region where S100+ and NeuN+ cells were scored. hGFAP-cre mice were crossed with ROSA26R mice, where Cre-mediated recombination drives the constitutive expression of the ß-galactosidase. X-gal staining positice cells are shown in blue. (C) Increase in cell cycle re-entry of FoxO null SVZ cells. Young mice (P8) were single-pulse labeled with BrdU 24hrs prior to the sacrifice and brain sections were stained for BrdU (red), Ki67 (green), and Sox2 (cyan). The fraction of Sox2 positive cells re-entered cell cycle (open arrow, BrdU+/Ki67+/Sox2+ triple positive) increased in FoxO null SVZ and that of no longer dividing (filled arrow, BrdU+/Ki67-/Sox2+) is higher in WT SVZ. Percent mean ±s.d. of Ki67+ cells from BrdU+/Sox2+ cells from 10 WT and 6 FoxO null mice is shown ( *, p<0.001 by two tail t-test). Bar=40μm (D) Ki67 positive NSC of FoxO WT and null cultures (n=5, P2). Bar=20μm.
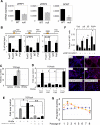
 )) mice SVZ were measured by laser scanning and plotted (4 pairs, *, p=0.004, **, p=0.0039, ‡, p=0.026, by two tail paired t-test). Decreased self-renewal capacity of NSC derived from 18-22 week old FoxO null mice (C) and acutely deleted for FoxOs in vitro (D). For conditional deletion NSC were isolated from SVZ of 4 week old Rosa26-CreERT2+ (null) or -(WT) FoxO1/3/4L/L mice and treated with 400nM 4OHT prior to the assay. Results are representative of three independent experiments with two primary cultures. Both average diameters for all the neurospheres and for multipotential ones are shown as mean±s.d. Proliferation index is shown as fraction of BrdU incorporation from triplicate cultures in two independent experiments. §, p=0.0043, #, p<0.0001, *, p=0.01, **, p=0.03, †, p=0.06, ‡, p=0.096 by two tail t-test. Bar= 100 μm.
)) mice SVZ were measured by laser scanning and plotted (4 pairs, *, p=0.004, **, p=0.0039, ‡, p=0.026, by two tail paired t-test). Decreased self-renewal capacity of NSC derived from 18-22 week old FoxO null mice (C) and acutely deleted for FoxOs in vitro (D). For conditional deletion NSC were isolated from SVZ of 4 week old Rosa26-CreERT2+ (null) or -(WT) FoxO1/3/4L/L mice and treated with 400nM 4OHT prior to the assay. Results are representative of three independent experiments with two primary cultures. Both average diameters for all the neurospheres and for multipotential ones are shown as mean±s.d. Proliferation index is shown as fraction of BrdU incorporation from triplicate cultures in two independent experiments. §, p=0.0043, #, p<0.0001, *, p=0.01, **, p=0.03, †, p=0.06, ‡, p=0.096 by two tail t-test. Bar= 100 μm.

 ) NSC compared to WT (■) . *, p=0.08053. (D) Attenuated Wnt3a-dependent canonical signaling by constitutively active FoxOs. CA1, CA3; FoxO1-ADA and FoxO3-AAA mutant respectively. *, p<0.1. (E) Enhanced canonical signaling in sFRP1/2 and SOST knockdown NSC (■) compared to WT (
) NSC compared to WT (■) . *, p=0.08053. (D) Attenuated Wnt3a-dependent canonical signaling by constitutively active FoxOs. CA1, CA3; FoxO1-ADA and FoxO3-AAA mutant respectively. *, p<0.1. (E) Enhanced canonical signaling in sFRP1/2 and SOST knockdown NSC (■) compared to WT ( ) is reversed by the addition of soluble sFRP1/2 and SOST (500ng/ml). *, p=0.068; **, p<0.005 (F) Exogenously added sFRP1/2 and SOST (500ng/ml) reversed increased proliferation of FoxO null NSC. Wnt3a, 50ng/ml of recombinant wnt3a was added for 24hrs. KD, 72 hrs after knockdown by siRNAs for sFRP1/2 and SOST. Fractions of Ki67 positive nuclei are plotted. (G) Decreased long term proliferation potential of NSC by Wnt3a stimulation (100ng/ml). Y axis indicates log2 (cells counted after 5 days in culture/cells seeded). Lines in red, FoxO null; blue, WT; yellow, Wnt3a treated. *, p=0.0041; **, p<0.01; #, p=0.0169.
) is reversed by the addition of soluble sFRP1/2 and SOST (500ng/ml). *, p=0.068; **, p<0.005 (F) Exogenously added sFRP1/2 and SOST (500ng/ml) reversed increased proliferation of FoxO null NSC. Wnt3a, 50ng/ml of recombinant wnt3a was added for 24hrs. KD, 72 hrs after knockdown by siRNAs for sFRP1/2 and SOST. Fractions of Ki67 positive nuclei are plotted. (G) Decreased long term proliferation potential of NSC by Wnt3a stimulation (100ng/ml). Y axis indicates log2 (cells counted after 5 days in culture/cells seeded). Lines in red, FoxO null; blue, WT; yellow, Wnt3a treated. *, p=0.0041; **, p<0.01; #, p=0.0169.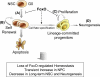
Comment in
-
Preview. The Tortoise, the hare, and the FoxO.Cell Stem Cell. 2009 Nov 6;5(5):451-2. doi: 10.1016/j.stem.2009.10.011. Cell Stem Cell. 2009. PMID: 19896431
References
-
- Alvarez B, Martinez AC, Burgering BM, Carrera AC. Forkhead transcription factors contribute to execution of the mitotic programme in mammals. Nature. 2001;413:744–747. - PubMed
-
- Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. - PubMed
-
- Birkenkamp KU, Essafi A, van der Vos KE, da Costa M, Hui RC, Holstege F, Koenderman L, Lam EW, Coffer PJ. FOXO3a induces differentiation of Bcr-Abl-transformed cells through transcriptional down-regulation of Id1. The Journal of biological chemistry. 2007;282:2211–2220. - PubMed
-
- Bond J, Roberts E, Mochida GH, Hampshire DJ, Scott S, Askham JM, Springell K, Mahadevan M, Crow YJ, Markham AF, et al. ASPM is a major determinant of cerebral cortical size. Nature genetics. 2002;32:316–320. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

