Dysmyelinated axons in shiverer mice are highly vulnerable to alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor-mediated toxicity
- PMID: 19896473
- PMCID: PMC7343376
- DOI: 10.1016/j.brainres.2009.10.066
Dysmyelinated axons in shiverer mice are highly vulnerable to alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor-mediated toxicity
Abstract
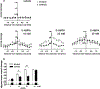
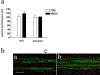
Glutamate excitotoxicity plays a role in white matter injury in many neurological diseases. Oligodendrocytes in particular are highly vulnerable to excitotoxicity, mediated through activation of AMPA/kainate receptors. Myelin may also be injured independently via NMDA (N-methyl-D-aspartic acid) receptors located on peripheral oligodendroglial processes. Central axons are susceptible to glutamate receptor activation in vivo, but it is unclear whether this is mediated directly by activation of receptors expressed on axons, or indirectly through glutamate toxicity of myelin or neighboring glial cells. We examined axonal vulnerability in mice deficient in myelin basic protein (shiverer), also expressing yellow fluorescent protein (YFP) in a subset of axons. YFP fluorescence, EM, and mouse behavior were assessed 24 h after microstereotactical injections of S-AMPA or NMDA into lumbar dorsal columns. S-AMPA injection led to impaired rotarod performance and widespread axonal degeneration and was more pronounced in shiverer mice than controls. In contrast, NMDA injection did not cause axonal injury or behavioral changes in either group. These results indicate that spinal cord axons in vivo are vulnerable to toxicity mediated by AMPA but not NMDA receptors. The presence of compact myelin is not required for excitotoxic axon damage, and its absence may increase vulnerability. Further understanding of AMPA receptor-mediated axonal toxicity may provide new targets for neuroprotective therapy in WM diseases.
Figures


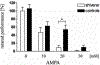

References
-
- Bannerman PG, Hahn A, 2007. Enhanced visualization of axonopathy in EAE using thy1-YFP transgenic mice. J Neurol Sci. 260, 23–32. - PubMed
-
- Beirowski B, Berek L, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP, 2004. Quantitative and qualitative analysis of Wallerian degeneration using restricted axonal labelling in YFP-H mice. J Neurosci Methods. 134, 23–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

