International collaboration between US and Thailand on a clinical trial of treatment for HIV-associated cryptococcal meningitis
- PMID: 19897055
- PMCID: PMC2861565
- DOI: 10.1016/j.cct.2009.11.002
International collaboration between US and Thailand on a clinical trial of treatment for HIV-associated cryptococcal meningitis
Abstract
Background: International clinical trials can provide scientific and logistic benefits in spite of the many challenges. Determining whether a country, especially a developing country, is an appropriate location for the research should include in-country consultation and partnering to assess its social value for the population; that treatments are relevant for the population under study; and that the research infrastructure and ethical oversight are adequate. Collaboration increases the likelihood of study success and helps ensure that benefits accrue to recruited populations and their community.
Purpose: This paper describes our experiences on a bi-national study and may provide guidance for those planning to engage in future collaborations.
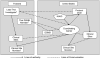
Methods: A Thai and United States team collaborated to develop and implement a phase II clinical trial for HIV-associated cryptococcal meningitis to assess safety and tolerability of combination therapy vs. standard treatment. Clinical and cultural differences, regulatory hurdles and operational issues were addressed before and during the study to ensure a successful collaboration between the 2 groups.
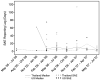
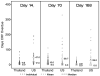
Results: The international multicenter study allowed for more rapid enrollment, reduced costs to complete the study, sharing of the benefits of research, greater generalizability of results and capacity building in Thailand; quality metrics in Thailand were equivalent to or better than those in the U.S.
Conclusions: Conducting successful clinical trials internationally requires early and ongoing collaboration to ensure the study meets sites' requirements and expectations, conforms to varying national regulations, adheres to data quality standards and is responsive to the health needs of studied populations.
Trial registration: ClinicalTrials.gov NCT00145249.
Copyright (c) 2009 Elsevier Inc. All rights reserved.
Conflict of interest statement
TL Nolen, LO Zimmer, S Pramanpol, ME Walker, D Wallace, P Pappas, P Chetchotisakd: No conflicts of interest
Figures

References
-
- Glickman SW, McHutchison JG, Peterson ED, Cairns CB, Harring RA, Califf RM, et al. Ethical and scientific implications of the globalization of clinical research. N Engl J Med. 2009;360(8):816–23. - PubMed
-
- Stough WG, Zannad F, Pitt B, Goldstein S. Globalization of cardiovascular clinical research: The balance between meeting medical needs and maintaining scientific standards. Am Heart J. 2007;154:232–8. - PubMed
-
- Emanuel EJ, Wendler D, Killen J, Grady C. What makes clinical research in developing countries ethical? The benchmarks of ethical research. JID. 2004;189:930–7. - PubMed
-
- Bicanic T, Harrison TS. Cryptococcal meningitis. Br Med Bull. 2005. [Accessed January 23, 2008.]. pp. 99–118. Available at: http://bmb.oxfordjournals.org/cgi/content/full/72/1/99. - PubMed
-
- Jones JL, Hanson DL, Dworkin MS, Alderton DL, Fleming PL, Kaplan JE, et al. MMWR Surveillance Summaries. Centers for Disease Control and Prevention Web site; [Accessed April 5, 2007.]. Surveillance for AIDS-Defining Opportunistic Illnesses, 1992–1997. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00056917.htm. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

