Factors influencing lopinavir and atazanavir plasma concentration
- PMID: 19897506
- PMCID: PMC2793688
- DOI: 10.1093/jac/dkp408
Factors influencing lopinavir and atazanavir plasma concentration
Abstract
Background: The protease inhibitors lopinavir and atazanavir are both recommended for treatment of HIV-infected patients. Considerable inter-individual variability in plasma concentration has been observed for both drugs. The aim of this study was to evaluate which demographic factors and concomitant drugs are associated with lopinavir and atazanavir plasma concentration.
Methods: Data from the Liverpool TDM (therapeutic drug monitoring) Registry were linked with the UK Collaborative HIV Cohort (CHIC) study. For each patient, the first measurement of lopinavir (twice daily) or atazanavir [once daily, ritonavir boosted (/r) or unboosted] plasma concentration was included. Linear regression was used to evaluate the association of dose, gender, age, weight, ethnicity and concomitant antiretroviral drugs or rifabutin with log-transformed drug concentration, adjusted for time since last intake.
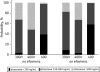
Results: Data from 439 patients on lopinavir (69% 400 mg/r, 31% 533 mg/r; 3% concomitant rifabutin) and 313 on atazanavir (60% 300 mg/r, 32% 400 mg/r, 8% 400 mg) were included. Multivariable models revealed the following predictors for lopinavir concentration: weight (11% decrease per additional 10 kg; P = 0.001); dose (25% increase for 533 mg/r; P = 0.024); and rifabutin (116% increase; P < 0.001). For atazanavir the predictors were dose (compared with 300 mg/r: 40% increase for 400 mg/r, 67% decrease for 400 mg; overall P < 0.001) and efavirenz (32% decrease; P = 0.016) but not tenofovir (P = 0.54).
Conclusions: This analysis confirms that efavirenz decreases atazanavir concentrations, and there was a negative association of weight and lopinavir concentrations. The strong impact of rifabutin on lopinavir concentration should be studied further.
Figures
References
-
- Gazzard BG. British HIV Association Guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Med. 2008;9:563–608. - PubMed
-
- Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–70. - PubMed
-
- Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372:646–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

