CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets
- PMID: 19897577
- PMCID: PMC2808153
- DOI: 10.1182/blood-2009-04-215491
CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets
Abstract
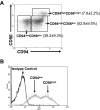
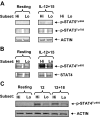
Human CD56(bright) natural killer (NK) cells possess little or no killer immunoglobulin-like receptors (KIRs), high interferon-gamma (IFN-gamma) production, but little cytotoxicity. CD56(dim) NK cells have high KIR expression, produce little IFN-gamma, yet display high cytotoxicity. We hypothesized that, if human NK maturation progresses from a CD56(bright) to a CD56(dim) phenotype, an intermediary NK cell must exist, which demonstrates more functional overlap than these 2 subsets, and we used CD94 expression to test our hypothesis. CD94(high)CD56(dim) NK cells express CD62L, CD2, and KIR at levels between CD56(bright) and CD94(low)CD56(dim) NK cells. CD94(high)CD56(dim) NK cells produce less monokine-induced IFN-gamma than CD56(bright) NK cells but much more than CD94(low)CD56(dim) NK cells because of differential interleukin-12-mediated STAT4 phosphorylation. CD94(high)CD56(dim) NK cells possess a higher level of granzyme B and perforin expression and CD94-mediated redirected killing than CD56(bright) NK cells but lower than CD94(low)CD56(dim) NK cells. Collectively, our data suggest that the density of CD94 surface expression on CD56(dim) NK cells identifies a functional and likely developmental intermediary between CD56(bright) and CD94(low)CD56(dim) NK cells. This supports the notion that, in vivo, human CD56(bright) NK cells progress through a continuum of differentiation that ends with a CD94(low)CD56(dim) phenotype.
Figures



References
-
- Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2(11):850–861. - PubMed
-
- Dorner BG, Smith HR, French AR, et al. Coordinate expression of cytokines and chemokines by NK cells during murine cytomegalovirus infection. J Immunol. 2004;172(5):3119–3131. - PubMed
-
- Luci C, Reynders A, Ivanov II, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10(1):75–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

