Proteomics-based discovery of a novel, structurally unique, and developmentally regulated plasminogen receptor, Plg-RKT, a major regulator of cell surface plasminogen activation
- PMID: 19897580
- PMCID: PMC2826757
- DOI: 10.1182/blood-2008-11-188938
Proteomics-based discovery of a novel, structurally unique, and developmentally regulated plasminogen receptor, Plg-RKT, a major regulator of cell surface plasminogen activation
Abstract
Activation of plasminogen, the zymogen of the primary thrombolytic enzyme, plasmin, is markedly promoted when plasminogen is bound to cell surfaces, arming cells with the broad spectrum proteolytic activity of plasmin. In addition to its role in thrombolysis, cell surface plasmin facilitates a wide array of physiologic and pathologic processes. Carboxypeptidase B-sensitive plasminogen binding sites promote plasminogen activation on eukaryotic cells. However, no integral membrane plasminogen receptors exposing carboxyl terminal basic residues on cell surfaces have been identified. Here we use the exquisite sensitivity of multidimensional protein identification technology and an inducible progenitor cell line to identify a novel differentiation-induced integral membrane plasminogen receptor that exposes a C-terminal lysine on the cell surface, Plg-R(KT) (C9orf46 homolog). Plg-R(KT) was highly colocalized on the cell surface with the urokinase receptor, uPAR. Our data suggest that Plg-R(KT) also interacts directly with tissue plasminogen activator. Furthermore, Plg-R(KT) markedly promoted cell surface plasminogen activation. Database searching revealed that Plg-R(KT) mRNA is broadly expressed by migratory cell types, including leukocytes, and breast cancer, leukemic, and neuronal cells. This structurally unique plasminogen receptor represents a novel control point for regulating cell surface proteolysis.
Figures
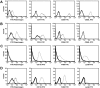
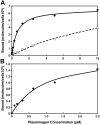


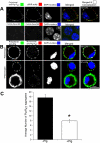
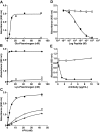
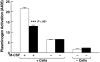
Comment in
-
A new plasminogen receptor.Blood. 2010 Feb 18;115(7):1315-6. doi: 10.1182/blood-2009-11-254045. Blood. 2010. PMID: 20167707 Free PMC article.
Similar articles
-
Functions of the plasminogen receptor Plg-RKT.J Thromb Haemost. 2020 Oct;18(10):2468-2481. doi: 10.1111/jth.15014. Epub 2020 Aug 19. J Thromb Haemost. 2020. PMID: 32662180 Free PMC article. Review.
-
The plasminogen receptor, Plg-R(KT), and macrophage function.J Biomed Biotechnol. 2012;2012:250464. doi: 10.1155/2012/250464. Epub 2012 Oct 14. J Biomed Biotechnol. 2012. PMID: 23125524 Free PMC article. Review.
-
New insights into the role of Plg-RKT in macrophage recruitment.Int Rev Cell Mol Biol. 2014;309:259-302. doi: 10.1016/B978-0-12-800255-1.00005-3. Int Rev Cell Mol Biol. 2014. PMID: 24529725 Free PMC article. Review.
-
Regulation of macrophage migration by a novel plasminogen receptor Plg-R KT.Blood. 2011 Nov 17;118(20):5622-30. doi: 10.1182/blood-2011-03-344242. Epub 2011 Sep 22. Blood. 2011. PMID: 21940822 Free PMC article.
-
The novel plasminogen receptor, plasminogen receptor(KT) (Plg-R(KT)), regulates catecholamine release.J Biol Chem. 2011 Sep 23;286(38):33125-33. doi: 10.1074/jbc.M111.218693. Epub 2011 Jul 27. J Biol Chem. 2011. PMID: 21795689 Free PMC article.
Cited by
-
Functions of the plasminogen receptor Plg-RKT.J Thromb Haemost. 2020 Oct;18(10):2468-2481. doi: 10.1111/jth.15014. Epub 2020 Aug 19. J Thromb Haemost. 2020. PMID: 32662180 Free PMC article. Review.
-
Location, location, location: Fibrin, cells, and fibrinolytic factors in thrombi.Front Cardiovasc Med. 2023 Jan 18;9:1070502. doi: 10.3389/fcvm.2022.1070502. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36741833 Free PMC article. Review.
-
Plasminogen Deficiency Delays the Onset and Protects from Demyelination and Paralysis in Autoimmune Neuroinflammatory Disease.J Neurosci. 2017 Apr 5;37(14):3776-3788. doi: 10.1523/JNEUROSCI.2932-15.2017. Epub 2017 Mar 8. J Neurosci. 2017. PMID: 28275164 Free PMC article.
-
The plasminogen receptor, Plg-R(KT), and macrophage function.J Biomed Biotechnol. 2012;2012:250464. doi: 10.1155/2012/250464. Epub 2012 Oct 14. J Biomed Biotechnol. 2012. PMID: 23125524 Free PMC article. Review.
-
A new plasminogen receptor.Blood. 2010 Feb 18;115(7):1315-6. doi: 10.1182/blood-2009-11-254045. Blood. 2010. PMID: 20167707 Free PMC article.
References
-
- Saksela O. Plasminogen activation and regulation of pericellular proteolysis. Biochim Biophys Acta. 1985;823(1):35–65. - PubMed
-
- Testa JE, Quigley JP. Protease receptors on cell surfaces: new mechanistic formulas applied to an old problem. J Natl Cancer Inst. 1988;80(10):712–713. - PubMed
-
- Ploplis VA, French EL, Carmeliet P, Collen D, Plow EF. Plasminogen deficiency differentially affects recruitment of inflammatory cell populations in mice. Blood. 1998;91(6):2005–2009. - PubMed
-
- Plow EF, Ploplis VA, Busuttil S, Carmeliet P, Collen D. A role of plasminogen in atherosclerosis and restenosis models in mice. Thromb Haemost. 1999;82(suppl 1):4–7. - PubMed
-
- Busuttil SJ, Ploplis VA, Castellino FJ, et al. A central role for plasminogen in the inflammatory response to biomaterials. J Thromb Haemost. 2004;2(10):1798–1805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

