A genomic island defines subspecies-specific virulence features of the host-adapted pathogen Campylobacter fetus subsp. venerealis
- PMID: 19897645
- PMCID: PMC2805309
- DOI: 10.1128/JB.00803-09
A genomic island defines subspecies-specific virulence features of the host-adapted pathogen Campylobacter fetus subsp. venerealis
Abstract
The pathogen Campylobacter fetus comprises two subspecies, C. fetus subsp. fetus and C. fetus subsp. venerealis. Although these taxa are highly related on the genome level, they are adapted to distinct hosts and tissues. C. fetus subsp. fetus infects a diversity of hosts, including humans, and colonizes the gastrointestinal tract. In contrast, C. fetus subsp. venerealis is largely restricted to the bovine genital tract, causing epidemic abortion in these animals. In light of their close genetic relatedness, the specific niche preferences make the C. fetus subspecies an ideal model system to investigate the molecular basis of host adaptation. In this study, a subtractive-hybridization approach was applied to the genomes of the subspecies to identify different genes potentially underlying this specificity. The comparison revealed a genomic island uniquely present in C. fetus subsp. venerealis that harbors several genes indicative of horizontal transfer and that encodes the core components necessary for bacterial type IV secretion. Macromolecular transporters of this type deliver effector molecules to host cells, thereby contributing to virulence in various pathogens. Mutational inactivation of the putative secretion system confirmed its involvement in the pathogenicity of C. fetus subsp. venerealis.
Figures



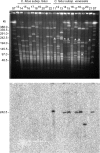


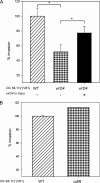
Similar articles
-
Genomic analysis of Campylobacter fetus subspecies: identification of candidate virulence determinants and diagnostic assay targets.BMC Microbiol. 2009 May 8;9:86. doi: 10.1186/1471-2180-9-86. BMC Microbiol. 2009. PMID: 19422718 Free PMC article.
-
Campylobacter fetus subspecies: comparative genomics and prediction of potential virulence targets.Gene. 2012 Oct 25;508(2):145-56. doi: 10.1016/j.gene.2012.07.070. Epub 2012 Aug 6. Gene. 2012. PMID: 22890137
-
Comparative genome analysis of Campylobacter fetus subspecies revealed horizontally acquired genetic elements important for virulence and niche specificity.PLoS One. 2014 Jan 9;9(1):e85491. doi: 10.1371/journal.pone.0085491. eCollection 2014. PLoS One. 2014. PMID: 24416416 Free PMC article.
-
So close and yet so far - Molecular Microbiology of Campylobacter fetus subspecies.Eur J Microbiol Immunol (Bp). 2012 Mar;2(1):66-75. doi: 10.1556/EuJMI.2.2012.1.10. Epub 2012 Mar 17. Eur J Microbiol Immunol (Bp). 2012. PMID: 24611123 Free PMC article. Review.
-
New molecular microbiology approaches in the study of Campylobacter fetus.Microb Biotechnol. 2011 Jan;4(1):8-19. doi: 10.1111/j.1751-7915.2010.00173.x. Microb Biotechnol. 2011. PMID: 21255368 Free PMC article. Review.
Cited by
-
Whole genome sequence analysis indicates recent diversification of mammal-associated Campylobacter fetus and implicates a genetic factor associated with H2S production.BMC Genomics. 2016 Sep 6;17(1):713. doi: 10.1186/s12864-016-3058-7. BMC Genomics. 2016. PMID: 27599479 Free PMC article.
-
Genomic investigation into strain heterogeneity and pathogenic potential of the emerging gastrointestinal pathogen Campylobacter ureolyticus.PLoS One. 2013 Aug 30;8(8):e71515. doi: 10.1371/journal.pone.0071515. eCollection 2013. PLoS One. 2013. PMID: 24023611 Free PMC article.
-
Application of direct polymerase chain reaction assays for Campylobacter fetus subsp. venerealis and Tritrichomonas foetus to screen preputial samples from breeding bulls in cow-calf herds in western Canada.Can J Vet Res. 2017 Apr;81(2):91-99. Can J Vet Res. 2017. PMID: 28408776 Free PMC article.
-
Campylobacter fetus Subspecies Contain Conserved Type IV Secretion Systems on Multiple Genomic Islands and Plasmids.PLoS One. 2016 Apr 6;11(4):e0152832. doi: 10.1371/journal.pone.0152832. eCollection 2016. PLoS One. 2016. PMID: 27049518 Free PMC article.
-
Two novel antibiotic resistance genes, tet(44) and ant(6)-Ib, are located within a transferable pathogenicity island in Campylobacter fetus subsp. fetus.Antimicrob Agents Chemother. 2010 Jul;54(7):3052-5. doi: 10.1128/AAC.00304-10. Epub 2010 May 17. Antimicrob Agents Chemother. 2010. PMID: 20479200 Free PMC article.
References
-
- Anonymous. 1996. OIE manual of standards for diagnostic tests and vaccines, 3rd ed., p. 256-266. Office International des Epizooties, Paris, France.
-
- Asakura, M., W. Samosornsuk, M. Taguchi, K. Kobayashi, N. Misawa, M. Kusumoto, K. Nishimura, A. Matsuhisa, and S. Yamasaki. 2007. Comparative analysis of cytolethal distending toxin (cdt) genes among Campylobacter jejuni, C. coli and C. fetus strains. Microb. Pathog. 42:174-183. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. A. Smith, J. G. Seidman, and K. Struhl. 1987. Current protocols in molecular biology. John Wiley & Sons, New York, NY.
-
- Baar, C., M. Eppinger, G. Raddatz, J. Simon, C. Lanz, O. Klimmek, R. Nandakumar, R. Gross, A. Rosinus, H. Keller, P. Jagtap, B. Linke, F. Meyer, H. Lederer, and S. C. Schuster. 2003. Complete genome sequence and analysis of Wolinella succinogenes. Proc. Natl. Acad. Sci. U. S. A. 100:11690-11695. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

