Low sensitivity of glucagon provocative testing for diagnosis of pheochromocytoma
- PMID: 19897672
- PMCID: PMC2805477
- DOI: 10.1210/jc.2009-1850
Low sensitivity of glucagon provocative testing for diagnosis of pheochromocytoma
Abstract
Context: Pheochromocytomas can usually be confirmed or excluded using currently available biochemical tests of catecholamine excess. Follow-up tests are, nevertheless, often required to distinguish false-positive from true-positive results. The glucagon stimulation test represents one such test; its diagnostic utility is, however, unclear.
Objective: The aim of the study was to determine the diagnostic power of the glucagon test to exclude or confirm pheochromocytoma.
Design, setting, and subjects: Glucagon stimulation tests were carried out at three specialist referral centers in 64 patients with pheochromocytoma, 38 patients in whom the tumor was excluded, and in a reference group of 36 healthy volunteers.
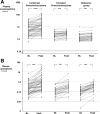
Main outcome measures: Plasma concentrations of norepinephrine and epinephrine were measured before and after glucagon administration. Several absolute and relative test criteria were used for calculating diagnostic sensitivity and specificity. Expression of the glucagon receptor was examined in pheochromocytoma tumor tissue from a subset of patients.
Results: Larger than 3-fold increases in plasma norepinephrine after glucagon strongly predicted the presence of a pheochromocytoma (100% specificity and positive predictive value). However, irrespective of the various criteria examined, glucagon-provoked increases in plasma catecholamines revealed the presence of the tumor in less than 50% of affected patients. Diagnostic sensitivity was particularly low in patients with pheochromocytomas due to von Hippel-Lindau syndrome. Tumors from these patients showed no significant expression of the glucagon receptor.
Conclusion: The glucagon stimulation test offers insufficient diagnostic sensitivity for reliable exclusion or confirmation of pheochromocytoma. Because of this and the risk of hypertensive complications, the test should be abandoned in routine clinical practice.
Figures


Similar articles
-
Plasma metanephrines in the diagnosis of pheochromocytoma.Ann Intern Med. 1995 Jul 15;123(2):101-9. doi: 10.7326/0003-4819-123-2-199507150-00004. Ann Intern Med. 1995. PMID: 7778821
-
[The glucagon test and diagnosis of pheochromocytoma].Schweiz Med Wochenschr. 1970 Jun 13;100(24):1023-6. Schweiz Med Wochenschr. 1970. PMID: 5524969 German. No abstract available.
-
Plasma norepinephrine and epinephrine responses to glucagon in patients with suspected pheochromocytomas.Metabolism. 1983 Oct;32(10):998-1001. doi: 10.1016/0026-0495(83)90142-7. Metabolism. 1983. PMID: 6888268 Clinical Trial.
-
New advances in the biochemical diagnosis of pheochromocytoma: moving beyond catecholamines.Ann N Y Acad Sci. 2002 Sep;970:29-40. doi: 10.1111/j.1749-6632.2002.tb04410.x. Ann N Y Acad Sci. 2002. PMID: 12381539 Review.
-
[Biological diagnosis of pheochromocytoma: impact of technological improvement].Ann Biol Clin (Paris). 1993;51(10-11):835-65. Ann Biol Clin (Paris). 1993. PMID: 8210060 Review. French.
Cited by
-
Metabologenomics of Phaeochromocytoma and Paraganglioma: An Integrated Approach for Personalised Biochemical and Genetic Testing.Clin Biochem Rev. 2017 Apr;38(2):69-100. Clin Biochem Rev. 2017. PMID: 29332973 Free PMC article. Review.
-
Pheochromocytoma and paraganglioma: diagnosis, genetics, management, and treatment.Curr Probl Cancer. 2014 Jan-Feb;38(1):7-41. doi: 10.1016/j.currproblcancer.2014.01.001. Epub 2014 Jan 15. Curr Probl Cancer. 2014. PMID: 24636754 Free PMC article. Review. No abstract available.
-
MYO5B mutations in pheochromocytoma/paraganglioma promote cancer progression.PLoS Genet. 2020 Jun 8;16(6):e1008803. doi: 10.1371/journal.pgen.1008803. eCollection 2020 Jun. PLoS Genet. 2020. PMID: 32511227 Free PMC article.
-
Pheochromocytoma in MEN2.Recent Results Cancer Res. 2025;223:211-235. doi: 10.1007/978-3-031-80396-3_8. Recent Results Cancer Res. 2025. PMID: 40102259 Review.
-
Pheochromocytoma and paraganglioma.Prog Brain Res. 2010;182:343-73. doi: 10.1016/S0079-6123(10)82015-1. Prog Brain Res. 2010. PMID: 20541673 Free PMC article.
References
-
- Lenders JW, Eisenhofer G, Mannelli M, Pacak K 2005 Pheochromocytoma. Lancet 366:665–675 - PubMed
-
- Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, Keiser HR, Goldstein DS, Eisenhofer G 2002 Biochemical diagnosis of pheochromocytoma: which test is best? JAMA 287:1427–1434 - PubMed
-
- Hickman PE, Leong M, Chang J, Wilson SR, McWhinney B 2009 Plasma free metanephrines are superior to urine and plasma catecholamines and urine catecholamine metabolites for the investigation of pheochromocytoma. Pathology 41:173–177 - PubMed
-
- Václavík J, Stejskal D, Lacnák B, Lazárová M, Jedelský L, Kadalová L, Janosová M, Frysák Z, Vlcek P 2007 Free plasma metanephrines as a screening test for pheochromocytoma in low-risk patients. J Hypertens 25:1427–1431 - PubMed
-
- Sawka AM, Jaeschke R, Singh RJ, Young Jr WF 2003 A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 88:553–558 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

