A genetically encoded reporter of synaptic activity in vivo
- PMID: 19898484
- PMCID: PMC2859341
- DOI: 10.1038/nmeth.1399
A genetically encoded reporter of synaptic activity in vivo
Abstract
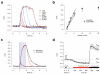
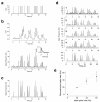
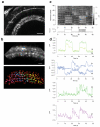
To image synaptic activity within neural circuits, we tethered the genetically encoded calcium indicator (GECI) GCaMP2 to synaptic vesicles by fusion to synaptophysin. The resulting reporter, SyGCaMP2, detected the electrical activity of neurons with two advantages over existing cytoplasmic GECIs: it identified the locations of synapses and had a linear response over a wider range of spike frequencies. Simulations and experimental measurements indicated that linearity arises because SyGCaMP2 samples the brief calcium transient passing through the presynaptic compartment close to voltage-sensitive calcium channels rather than changes in bulk calcium concentration. In vivo imaging in zebrafish demonstrated that SyGCaMP2 can assess electrical activity in conventional synapses of spiking neurons in the optic tectum and graded voltage signals transmitted by ribbon synapses of retinal bipolar cells. Localizing a GECI to synaptic terminals provides a strategy for monitoring activity across large groups of neurons at the level of individual synapses.
Figures



Comment in
-
Lighting up neural networks using a new generation of genetically encoded calcium sensors.Nat Methods. 2009 Dec;6(12):871-2. doi: 10.1038/nmeth1209-871. Nat Methods. 2009. PMID: 19935839
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

