Rat Merkel cells are mechanoreceptors and osmoreceptors
- PMID: 19898622
- PMCID: PMC2770322
- DOI: 10.1371/journal.pone.0007759
Rat Merkel cells are mechanoreceptors and osmoreceptors
Abstract
Merkel cells (MCs) associated with nerve terminals constitute MC-neurite complexes, which are involved in slowly-adapting type I mechanoreception. Although MCs are known to express voltage-gated Ca2+ channels and hypotonic-induced membrane deformation is known to lead to Ca2+ transients, whether MCs initiate mechanotransduction is currently unknown. To answer to this question, rat MCs were transfected with a reporter vector, which enabled their identification.Their properties were investigated through electrophysiological studies. Voltage-gated K+, Ca2+ and Ca2+-activated K+ (KCa)channels were identified, as previously described. Here, we also report the activation of Ca2+ channels by histamine and their inhibition by acetylcholine. As a major finding, we demonstrated that direct mechanical stimulations induced strong inward Ca2+ currents in MCs. Depolarizations were dependent on the strength and the length of the stimulation. Moreover, touch-evoked currents were inhibited by the stretch channel antagonist gadolinium. These data confirm the mechanotransduction capabilities of MCs. Furthermore, we found that activation of the osmoreceptor TRPV4 in FM1-43-labeled MCs provoked neurosecretory granule exocytosis. Since FM1-43 blocks mechanosensory channels, this suggests that hypo-osmolarity activates MCs in the absence of mechanotransduction. Thus, mechanotransduction and osmoreception are likely distinct pathways.
Conflict of interest statement
Figures


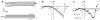




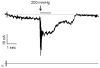



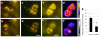
References
-
- Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. - PubMed
-
- Johnson KO. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol. 2001;11:455–461. - PubMed
-
- Boulais N, Misery L. Merkel cells. J Am Acad Dermatol. 2007;57:147–165. - PubMed
-
- Gaudillere A, Misery L. Merkel cell. Ann Dermatol Venereol. 1994;121:909–917. - PubMed
-
- Mills LR, Diamond J. Merkel cells are not the mechanosensory transducers in the touch dome of the rat. J Neurocytol. 1995;24:117–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

