Investigating sub-spine actin dynamics in rat hippocampal neurons with super-resolution optical imaging
- PMID: 19898630
- PMCID: PMC2771285
- DOI: 10.1371/journal.pone.0007724
Investigating sub-spine actin dynamics in rat hippocampal neurons with super-resolution optical imaging
Abstract
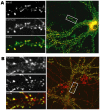
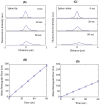
Morphological changes in dendritic spines represent an important mechanism for synaptic plasticity which is postulated to underlie the vital cognitive phenomena of learning and memory. These morphological changes are driven by the dynamic actin cytoskeleton that is present in dendritic spines. The study of actin dynamics in these spines traditionally has been hindered by the small size of the spine. In this study, we utilize a photo-activation localization microscopy (PALM)-based single-molecule tracking technique to analyze F-actin movements with approximately 30-nm resolution in cultured hippocampal neurons. We were able to observe the kinematic (physical motion of actin filaments, i.e., retrograde flow) and kinetic (F-actin turn-over) dynamics of F-actin at the single-filament level in dendritic spines. We found that F-actin in dendritic spines exhibits highly heterogeneous kinematic dynamics at the individual filament level, with simultaneous actin flows in both retrograde and anterograde directions. At the ensemble level, movements of filaments integrate into a net retrograde flow of approximately 138 nm/min. These results suggest a weakly polarized F-actin network that consists of mostly short filaments in dendritic spines.
Conflict of interest statement
Figures





References
-
- Matus A. Actin-Based Plasticity in Dendritic Spines. Science. 2000;290:754–758. - PubMed
-
- Parnass Z, Tashiro A, Yuste R. Analysis of spine morphological plasticity in developing hippocampal pyramidal neurons. Hippocampus. 2000;10:561–568. - PubMed
-
- Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

