Protein myristoylation in health and disease
- PMID: 19898886
- PMCID: PMC2816741
- DOI: 10.1007/s12154-009-0032-8
Protein myristoylation in health and disease
Abstract
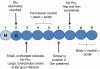
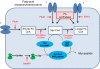
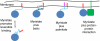
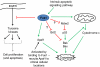
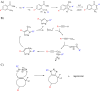
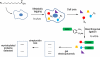
N-myristoylation is the attachment of a 14-carbon fatty acid, myristate, onto the N-terminal glycine residue of target proteins, catalysed by N-myristoyltransferase (NMT), a ubiquitous and essential enzyme in eukaryotes. Many of the target proteins of NMT are crucial components of signalling pathways, and myristoylation typically promotes membrane binding that is essential for proper protein localisation or biological function. NMT is a validated therapeutic target in opportunistic infections of humans by fungi or parasitic protozoa. Additionally, NMT is implicated in carcinogenesis, particularly colon cancer, where there is evidence for its upregulation in the early stages of tumour formation. However, the study of myristoylation in all organisms has until recently been hindered by a lack of techniques for detection and identification of myristoylated proteins. Here we introduce the chemistry and biology of N-myristoylation and NMT, and discuss new developments in chemical proteomic technologies that are meeting the challenge of studying this important co-translational modification in living systems.
Figures



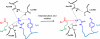




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

