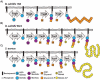
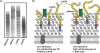
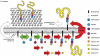
Wall teichoic acid function, biosynthesis, and inhibition
- PMID: 19899094
- PMCID: PMC2798926
- DOI: 10.1002/cbic.200900557
Wall teichoic acid function, biosynthesis, and inhibition
Figures



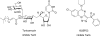

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

