Functional morphology of the sound-generating labia in the syrinx of two songbird species
- PMID: 19900184
- PMCID: PMC2807973
- DOI: 10.1111/j.1469-7580.2009.01161.x
Functional morphology of the sound-generating labia in the syrinx of two songbird species
Abstract
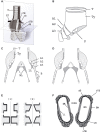
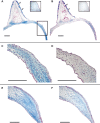
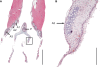
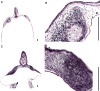
In songbirds, two sound sources inside the syrinx are used to produce the primary sound. Laterally positioned labia are passively set into vibration, thus interrupting a passing air stream. Together with subsyringeal pressure, the size and tension of the labia determine the spectral characteristics of the primary sound. Very little is known about how the histological composition and morphology of the labia affect their function as sound generators. Here we related the size and microstructure of the labia to their acoustic function in two songbird species with different acoustic characteristics, the white-crowned sparrow and zebra finch. Histological serial sections of the syrinx and different staining techniques were used to identify collagen, elastin and hyaluronan as extracellular matrix components. The distribution and orientation of elastic fibers indicated that the labia in white-crowned sparrows are multi-layered structures, whereas they are more uniformly structured in the zebra finch. Collagen and hyaluronan were evenly distributed in both species. A multi-layered composition could give rise to complex viscoelastic properties of each sound source. We also measured labia size. Variability was found along the dorso-ventral axis in both species. Lateral asymmetry was identified in some individuals but not consistently at the species level. Different size between the left and right sound sources could provide a morphological basis for the acoustic specialization of each sound generator, but only in some individuals. The inconsistency of its presence requires the investigation of alternative explanations, e.g. differences in viscoelastic properties of the labia of the left and right syrinx. Furthermore, we identified attachments of syringeal muscles to the labia as well as to bronchial half rings and suggest a mechanism for their biomechanical function.
Figures



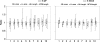

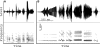
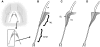
References
-
- Amador A, Goller F, Mindlin GB. Frequency modulation during song in a suboscine does not require vocal muscles. J Neurophysiol. 2008;99:2383–2389. - PubMed
-
- Ames PL. The morphology of the syrinx in passerine birds. New Haven, Connecticut: Peabody Museum of Natural History, Yale University; 1971.
-
- Caton TBS, Thibeault SL, Klemuk S, et al. Viscoelasticity of hyaluronan and nonhyaluronan based vocal fold injectables: implications for mucosal versus muscle use. Laryngoscope. 2007;117:516–521. - PubMed
-
- Chamberlain DR, Gross WB, Cornwell GW, et al. Syringeal anatomy in the common crow. Auk. 1968;85:244–252.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

