Biliary acute pancreatitis in mice is mediated by the G-protein-coupled cell surface bile acid receptor Gpbar1
- PMID: 19900448
- PMCID: PMC2819588
- DOI: 10.1053/j.gastro.2009.10.052
Biliary acute pancreatitis in mice is mediated by the G-protein-coupled cell surface bile acid receptor Gpbar1
Abstract
Background & aims: The mechanisms by which reflux of bile acids into the pancreas induces pancreatitis are unknown. We reasoned that key events responsible for this phenomenon might be mediated by Gpbar1, a recently identified and widely expressed G-protein-coupled, cell surface bile acid receptor.
Methods: Acute pancreatitis was induced in wild-type and Gpbar1(-/-) mice by either retrograde ductal infusion of taurolithocholic acid-3-sulfate (TLCS) or supramaximal secretagogue stimulation with caerulein. In vitro experiments were performed in which acini obtained from wild-type and Gpbar1(-/-) mice were exposed to either submicellar concentrations of TLCS (200-500 microM) or a supramaximally stimulating concentration of caerulein (10 nM).
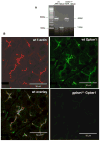
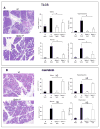
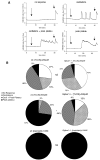
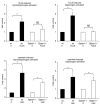
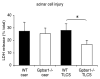
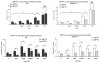
Results: Gpbar1 is expressed at the apical pole of acinar cells and its genetic deletion is associated with reduced hyperamylasemia, edema, inflammation, and acinar cell injury in TLCS-induced, but not caerulein-induced, pancreatitis. In vitro, genetic deletion of Gpbar1 is associated with markedly reduced generation of pathological calcium transients, intracellular activation of digestive zymogens, and cell injury when these responses are induced by exposure to TLCS, but not when they are induced by exposure to caerulein.
Conclusions: Gpbar1 may play a critical role in the evolution of bile-acid-induced pancreatitis by coupling exposure to bile acids with generation of pathological intracellular calcium transients, intra-acinar cell zymogen activation, and acinar cell injury. Acute biliary pancreatitis may be a "receptor-mediated" disease and interventions that interfere with Gpbar1 function might prove beneficial in the treatment and/or prevention of biliary acute pancreatitis.
Figures
Comment in
-
The role of bile acids in gallstone-induced pancreatitis.Gastroenterology. 2010 Feb;138(2):429-33. doi: 10.1053/j.gastro.2009.12.012. Epub 2009 Dec 22. Gastroenterology. 2010. PMID: 20034603 No abstract available.
Similar articles
-
Review of experimental animal models of biliary acute pancreatitis and recent advances in basic research.HPB (Oxford). 2012 Feb;14(2):73-81. doi: 10.1111/j.1477-2574.2011.00408.x. HPB (Oxford). 2012. PMID: 22221567 Free PMC article. Review.
-
Bile acids induce pancreatic acinar cell injury and pancreatitis by activating calcineurin.J Biol Chem. 2013 Jan 4;288(1):570-80. doi: 10.1074/jbc.M112.428896. Epub 2012 Nov 12. J Biol Chem. 2013. PMID: 23148215 Free PMC article.
-
Cholic acid activation of GPBAR1 does not induce or exacerbate acute pancreatitis but promotes exocrine pancreatic secretion.Biochem Biophys Res Commun. 2024 Nov 26;735:150825. doi: 10.1016/j.bbrc.2024.150825. Epub 2024 Oct 12. Biochem Biophys Res Commun. 2024. PMID: 39426134
-
TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis.Gastroenterology. 2011 Jul;141(1):358-69. doi: 10.1053/j.gastro.2011.03.041. Epub 2011 Mar 24. Gastroenterology. 2011. PMID: 21439959 Free PMC article.
-
Role of Bile Acids and Bile Salts in Acute Pancreatitis: From the Experimental to Clinical Studies.Pancreas. 2021 Jan 1;50(1):3-11. doi: 10.1097/MPA.0000000000001706. Pancreas. 2021. PMID: 33370017 Free PMC article. Review.
Cited by
-
Acute Pancreatitis: Diagnosis and Treatment.Drugs. 2022 Aug;82(12):1251-1276. doi: 10.1007/s40265-022-01766-4. Epub 2022 Sep 8. Drugs. 2022. PMID: 36074322 Free PMC article. Review.
-
Molecular and cellular mechanisms of pancreatic injury.Curr Opin Gastroenterol. 2010 Sep;26(5):484-9. doi: 10.1097/MOG.0b013e32833d119e. Curr Opin Gastroenterol. 2010. PMID: 20651589 Free PMC article. Review.
-
Review of experimental animal models of biliary acute pancreatitis and recent advances in basic research.HPB (Oxford). 2012 Feb;14(2):73-81. doi: 10.1111/j.1477-2574.2011.00408.x. HPB (Oxford). 2012. PMID: 22221567 Free PMC article. Review.
-
Combination of the CRAC Channel Inhibitor CM4620 and Galactose as a Potential Therapy for Acute Pancreatitis.Function (Oxf). 2024 Jul 11;5(4):zqae017. doi: 10.1093/function/zqae017. Function (Oxf). 2024. PMID: 38984998 Free PMC article.
-
Pathogenic mechanisms of acute pancreatitis.Curr Opin Gastroenterol. 2012 Sep;28(5):507-15. doi: 10.1097/MOG.0b013e3283567f52. Curr Opin Gastroenterol. 2012. PMID: 22885948 Free PMC article. Review.
References
-
- Opie EL. The etiology of acute hemorrhagic pancreatitis. Bull Johns Hopkins Hosp. 1901;12:182–8.
-
- Opie EL. The relationship of cholelithiasis to disease of the pancreas and fat necrosis. Bull Johns Hopkins Hosp. 1901;12:19–21.
-
- Lerch MM, Saluja AK, Runzi M, et al. Pancreatic duct obstruction triggers acute necrotizing pancreatitis in the opossum. Gastroenterology. 1993;104:853–861. - PubMed
-
- Aho HJ, Koskensalo SM, Nevalainen TJ. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute hemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15:411–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

