Clonal analysis and hierarchy of human bone marrow mesenchymal stem and progenitor cells
- PMID: 19900502
- PMCID: PMC2807618
- DOI: 10.1016/j.exphem.2009.11.001
Clonal analysis and hierarchy of human bone marrow mesenchymal stem and progenitor cells
Abstract
Objective: This study was performed to assess adult human bone marrow mesenchymal stem/progenitor cells at a single-cell level and to determine a hierarchy based on proliferative potential.
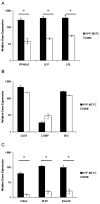
Materials and methods: Adult bone marrow mesenchymal cells expressing the enhanced green fluorescent protein (EGFP) were sorted as single cells into 24-well plates, each well confirmed with single EGFP-positive cells by fluorescence microscopy, and counted every 3 days. Colonies derived from single cells were expanded then sorted and evaluated using established differentiation protocols for adipogenic, chondrogenic, and osteogenic lineages. Cells were further analyzed by real-time reverse transcription polymerase chain reaction (RT-PCR) (peroxisome proliferator-activated receptor[PPAR]-gamma2, LEP, LPL, LUM, COMP, BIG, RUNX2, IBSP, BGLAP) and immunocytochemistry (PPAR-gamma1/2, collagen II, bone sialoprotein II) specific for trilineage differentiation.
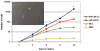
Results: Bone marrow mesenchymal cells were found to contain high proliferative potential (HPP) mesenchymal colony-forming cells (MCFC) (7%), low proliferative potential (LPP) MCFC (29%), mesenchymal cell clusters (MCC, 26%), and mature mesenchymal cells (MMC, 38%). All LPP-MCFC, MCC, and MMC colonies reached senescence at the end of the evaluation period. However, HPP-MCFC continued to grow, showed differentiation toward all three lineages, and demonstrated the capacity to give rise to secondary HPP-MCFC upon replating at a clonal level.
Conclusion: These findings suggest that there is a low frequency of bone marrow-derived HPP-MCFC that can both self-renew at a single-cell level and differentiate toward multiple lineages of mesenchymal origin.
Figures



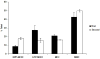

References
-
- McNiece IK, Bertoncello I, Kriegler AB, Quesenberry PJ. Colony-forming cells with high proliferative potential (HPP-CFC) Int J Cell Cloning. 1990;8:146–160. - PubMed
-
- Yoder MC, Du XX, Williams DA. High proliferative potential colony-forming cell heterogeneity identified using counterflow centrifugal elutriation. Blood. 1993;82:385–391. - PubMed
-
- Bradley TR, Hodgson GS. Detection of primitive macrophage progenitor cells in mouse bone marrow. Blood. 1979;54:1446–1450. - PubMed
-
- Srour EF, Brandt JE, Briddell RA, Grigsby S, Leemhuis T, Hoffman R. Long-term generation and expansion of human primitive hematopoietic progenitor cells in vitro. Blood. 1993;81:661–669. - PubMed
-
- Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

