Postsynaptic PDLIM5/Enigma Homolog binds SPAR and causes dendritic spine shrinkage
- PMID: 19900557
- PMCID: PMC2818455
- DOI: 10.1016/j.mcn.2009.10.009
Postsynaptic PDLIM5/Enigma Homolog binds SPAR and causes dendritic spine shrinkage
Abstract
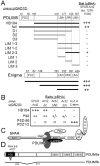
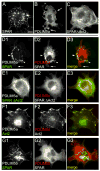
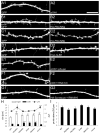
Dendritic spine morphology is thought to play important roles in synaptic development and plasticity, and morphological derangements in spines are correlated with several neurological disorders. Here, we identified an interaction between Spine-Associated RapGAP (SPAR), a postsynaptic protein that reorganizes actin cytoskeleton and drives dendritic spine head growth, and PDLIM5/Enigma Homolog (ENH), a PDZ-LIM (postsynaptic density-95/Discs large/zona occludens 1-Lin11/Isl-1/Mec3) family member. PDLIM5 has been implicated in susceptibility to bipolar disorder, major depression, and schizophrenia, but its function in neurological disease is poorly understood. We show that PDLIM5 is present in the postsynaptic density, where it promotes decreased dendritic spine head size and longer, filopodia-like morphology. Conversely, RNA interference against PDLIM5 or loss of PDLIM5 interaction with SPAR caused increased spine head diameter. Furthermore, PKC activation promoted delivery of PDLIM5 into dendritic spines and increased its spine colocalization with SPAR. These data reveal new postsynaptic functions for PDLIM5 in shrinkage of dendritic spines that may be relevant to its association with psychiatric illness.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP.Neuron. 2001 Aug 2;31(2):289-303. doi: 10.1016/s0896-6273(01)00355-5. Neuron. 2001. PMID: 11502259
-
Coordination of synaptic adhesion with dendritic spine remodeling by AF-6 and kalirin-7.J Neurosci. 2008 Jun 11;28(24):6079-91. doi: 10.1523/JNEUROSCI.1170-08.2008. J Neurosci. 2008. PMID: 18550750 Free PMC article.
-
NMDA receptor-dependent regulation of dendritic spine morphology by SAP102 splice variants.J Neurosci. 2011 Jan 5;31(1):89-96. doi: 10.1523/JNEUROSCI.1034-10.2011. J Neurosci. 2011. PMID: 21209193 Free PMC article.
-
Combinatorial morphogenesis of dendritic spines and filopodia by SPAR and alpha-actinin2.Biochem Biophys Res Commun. 2009 Jun 19;384(1):55-60. doi: 10.1016/j.bbrc.2009.04.069. Epub 2009 Apr 23. Biochem Biophys Res Commun. 2009. PMID: 19393616 Free PMC article.
-
Glutamatergic postsynaptic density protein dysfunctions in synaptic plasticity and dendritic spines morphology: relevance to schizophrenia and other behavioral disorders pathophysiology, and implications for novel therapeutic approaches.Mol Neurobiol. 2014 Feb;49(1):484-511. doi: 10.1007/s12035-013-8534-3. Epub 2013 Sep 3. Mol Neurobiol. 2014. PMID: 23999870 Review.
Cited by
-
Exploring the PDZ, DUF, and LIM Domains of Pdlim5 in Dendrite Branching.Int J Mol Sci. 2024 Jul 30;25(15):8326. doi: 10.3390/ijms25158326. Int J Mol Sci. 2024. PMID: 39125895 Free PMC article.
-
Transcriptional analysis of apoptotic cerebellar granule neurons following rescue by gastric inhibitory polypeptide.Int J Mol Sci. 2014 Apr 1;15(4):5596-622. doi: 10.3390/ijms15045596. Int J Mol Sci. 2014. PMID: 24694544 Free PMC article.
-
Transcriptomics reveals amygdala neuron regulation by fasting and ghrelin thereby promoting feeding.Sci Adv. 2023 May 24;9(21):eadf6521. doi: 10.1126/sciadv.adf6521. Epub 2023 May 24. Sci Adv. 2023. PMID: 37224253 Free PMC article.
-
Novel phospho-switch function of delta-catenin in dendrite development.J Cell Biol. 2020 Nov 2;219(11):e201909166. doi: 10.1083/jcb.201909166. J Cell Biol. 2020. PMID: 33007084 Free PMC article.
-
A novel effect of PDLIM5 in α7 nicotinic acetylcholine receptor upregulation and surface expression.Cell Mol Life Sci. 2022 Jan 10;79(1):64. doi: 10.1007/s00018-021-04115-y. Cell Mol Life Sci. 2022. PMID: 35013841 Free PMC article.
References
-
- Abeliovich A, Chen C, Goda Y, Silva AJ, Stevens CF, Tonegawa S. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell. 1993;75:1253–1262. - PubMed
-
- Arinami T. Initial genome-wide scan for linkage with schizophrenia in the Japanese Schizophrenia Sib-Pair Linkage Group (JSSLG) families. Am J Med Genet B Neuropsychiatr Genet. 2003;120B:22–28. - PubMed
-
- Blanpied TA, Ehlers MD. Microanatomy of dendritic spines: emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry. 2004;55:1121–1127. - PubMed
-
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

