Increased Ca(2+) leak and spatiotemporal coherence of Ca(2+) release in cardiomyocytes during beta-adrenergic stimulation
- PMID: 19900959
- PMCID: PMC2821561
- DOI: 10.1113/jphysiol.2009.181800
Increased Ca(2+) leak and spatiotemporal coherence of Ca(2+) release in cardiomyocytes during beta-adrenergic stimulation
Abstract
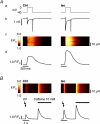
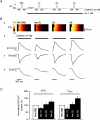
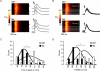
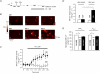
beta-Adrenergic receptor (beta-AR) stimulation of cardiac muscle has been proposed to enhance Ca(2+) release from the sarcoplasmic reticulum (SR) through ryanodine receptors (RyRs). However, the anticipated increase in RyR Ca(2+) sensitivity has proven difficult to study in intact cardiomyocytes, due to accompanying alterations in SR Ca(2+) content, inward Ca(2+) current (I(Ca)) and diastolic cytosolic Ca(2+) concentration ([Ca(2+)](i)). Here, we studied whole-cell Ca(2+) release and spontaneous Ca(2+) leak (Ca(2+) sparks) in guinea-pig ventricular myocytes with confocal Ca(2+) imaging before and during beta-AR stimulation by isoproterenol (Iso), but under otherwise nearly identical experimental conditions. The extent of SR Ca(2+) loading was controlled under whole-cell voltage-clamp conditions. UV flash-induced uncaging of Ca(2+) from DM-nitrophen was employed as an invariant trigger for whole-cell Ca(2+) release. At matched SR Ca(2+) content, we found that Iso enhanced the spatiotemporal coherence of whole-cell Ca(2+) release, evident from spatially intercorrelated release and accelerated release kinetics that resulted in moderately (20%) increased release amplitude. This may arise from higher RyR Ca(2+) sensitivity, and was also reflected in spontaneous SR Ca(2+) leak. At comparable SR Ca(2+) content and cytosolic [Ca(2+)](i), we observed an approximately 4-fold increase in Ca(2+) spark frequency in Iso that also appeared in quiescent cells within 2 min without increased SR Ca(2+) content. This was likely to have been mediated by Ca(2+)/calmodulin-dependent protein kinase (CaMKII), rather than cAMP dependent protein kinase (PKA). We conclude that Iso increases the propensity of RyRs to open, both in response to rapid elevations of [Ca(2+)](i) and at diastolic [Ca(2+)](i). While this could be beneficial in enhancing and synchronizing systolic whole-cell SR Ca(2+) release, the same behaviour could also be proarrhythmogenic during diastole.
Figures




Comment in
-
Stress synchronizes calcium release and promotes SR calcium leak.J Physiol. 2010 Feb 1;588(Pt 3):391-2. doi: 10.1113/jphysiol.2009.184978. J Physiol. 2010. PMID: 20123791 Free PMC article. No abstract available.
Similar articles
-
Reactive oxygen species contribute to the development of arrhythmogenic Ca²⁺ waves during β-adrenergic receptor stimulation in rabbit cardiomyocytes.J Physiol. 2012 Jul 15;590(14):3291-304. doi: 10.1113/jphysiol.2012.230748. Epub 2012 May 14. J Physiol. 2012. PMID: 22586224 Free PMC article.
-
Calcium/calmodulin-dependent kinase II and nitric oxide synthase 1-dependent modulation of ryanodine receptors during β-adrenergic stimulation is restricted to the dyadic cleft.J Physiol. 2016 Oct 15;594(20):5923-5939. doi: 10.1113/JP271965. Epub 2016 Jul 3. J Physiol. 2016. PMID: 27121757 Free PMC article.
-
Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase.Circ Res. 2007 Feb 16;100(3):391-8. doi: 10.1161/01.RES.0000258172.74570.e6. Epub 2007 Jan 18. Circ Res. 2007. PMID: 17234966
-
Rhythmic Ca2+ oscillations drive sinoatrial nodal cell pacemaker function to make the heart tick.Ann N Y Acad Sci. 2005 Jun;1047:138-56. doi: 10.1196/annals.1341.013. Ann N Y Acad Sci. 2005. PMID: 16093492 Review.
-
Beta-adrenergic receptor signaling in the heart: role of CaMKII.J Mol Cell Cardiol. 2010 Feb;48(2):322-30. doi: 10.1016/j.yjmcc.2009.10.016. Epub 2009 Oct 31. J Mol Cell Cardiol. 2010. PMID: 19883653 Free PMC article. Review.
Cited by
-
Reactive oxygen species contribute to the development of arrhythmogenic Ca²⁺ waves during β-adrenergic receptor stimulation in rabbit cardiomyocytes.J Physiol. 2012 Jul 15;590(14):3291-304. doi: 10.1113/jphysiol.2012.230748. Epub 2012 May 14. J Physiol. 2012. PMID: 22586224 Free PMC article.
-
β-Adrenergic stimulation increases the intra-sarcoplasmic reticulum Ca2+ threshold for Ca2+ wave generation.J Physiol. 2012 Dec 1;590(23):6093-108. doi: 10.1113/jphysiol.2012.236117. Epub 2012 Sep 17. J Physiol. 2012. PMID: 22988136 Free PMC article.
-
Epac2 mediates cardiac β1-adrenergic-dependent sarcoplasmic reticulum Ca2+ leak and arrhythmia.Circulation. 2013 Feb 26;127(8):913-22. doi: 10.1161/CIRCULATIONAHA.12.148619. Epub 2013 Jan 30. Circulation. 2013. PMID: 23363625 Free PMC article.
-
Preventing the phosphorylation of RyR2 at canonical sites reduces Ca2+ leak and promotes arrhythmia by reactivating the INa current.Nat Cardiovasc Res. 2025 Aug;4(8):976-990. doi: 10.1038/s44161-025-00693-3. Epub 2025 Aug 12. Nat Cardiovasc Res. 2025. PMID: 40797044 Free PMC article.
-
Dysfunctional ryanodine receptors in the heart: new insights into complex cardiovascular diseases.J Mol Cell Cardiol. 2013 May;58:225-31. doi: 10.1016/j.yjmcc.2013.03.005. Epub 2013 Mar 16. J Mol Cell Cardiol. 2013. PMID: 23507255 Free PMC article. Review.
References
-
- Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. - PubMed
-
- Altamirano J, Bers DM. Voltage dependence of cardiac excitation-contraction coupling: unitary Ca2+ current amplitude and open channel probability. Circ Res. 2007;101:590–597. - PubMed
-
- Barrett SD. Software for scanning microscopy. Proc Roy Microsc Soc. 2002;37:164–174.
-
- Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, Powers PA, Valdivia HH. Intact β-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ Res. 2007;101:819–829. - PubMed
-
- Berlin JR, Cannell MB, Lederer WJ. Cellular origins of the transient inward current in cardiac myocytes. Role of fluctuations and waves of elevated intracellular calcium. Circ Res. 1989;65:115–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

