Excitability parameters and sensitivity to anemone toxin ATX-II in rat small diameter primary sensory neurones discriminated by Griffonia simplicifolia isolectin IB4
- PMID: 19900960
- PMCID: PMC2821554
- DOI: 10.1113/jphysiol.2009.181107
Excitability parameters and sensitivity to anemone toxin ATX-II in rat small diameter primary sensory neurones discriminated by Griffonia simplicifolia isolectin IB4
Abstract
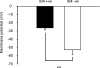
Sensory neurone subtypes (< or = 25 microm apparent diameter) express a variety of Na(+) channels, where expression is linked to action potential duration, and associated with differential IB4-lectin binding. We hypothesized that sensitivity to ATX-II might also discriminate neurones and report that 1 microm has negligible or small effects on action potentials in IB4 +ve, but dramatically increased action potential duration in IB4 ve, neurones. The toxin did not act on tetrodotoxin-resistant (TTX-r) Na(V)1.8 currents; discrimination was based on tetrodotoxin-sensitive (TTX-s) Na(+) channel expression. We also explored the effects of varying the holding potential on current threshold, and the effect of repetitive activation on action currents in IB4 +ve and ve neurones. IB4 +ve neurones became more excitable with depolarization over the range 100 to 20 mV, but IB4 ve neurones exhibited peak excitability near 55 mV, and were inexcitable at 20 mV. Eliciting action potentials at 2 Hz, we found that peak inward action current in IB4 +ve neurones was reduced, whereas changes in the current amplitude were negligible in most IB4 ve neurones. Our findings are consistent with relatively toxin-insensitive channels including Na(V)1.7 being expressed in IB4 +ve neurones, whereas toxin sensitivity indicates that IB4 ve neurones may express Na(V)1.1 or Na(V)1.2, or both. The retention of excitability at low membrane potentials, and the responses to repetitive stimulation are explained by the known preferential expression of Na(V)1.8 in IB4 +ve neurones, and the reduction in action current in IB4 +ve neurones with repetitive stimulation supports a novel hypothesis explaining the slowing of conduction velocity in C-fibres by the build-up of Na(+) channel inactivation.
Figures

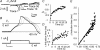





Comment in
-
Sea anemone 'sting' isolates IB4-negative sensory neurones.J Physiol. 2010 Jan 1;588(Pt 1):11. doi: 10.1113/jphysiol.2009.185017. J Physiol. 2010. PMID: 20045899 Free PMC article. No abstract available.
References
-
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. - PubMed
-
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
-
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

