Baculovirus-based nasal drop vaccine confers complete protection against malaria by natural boosting of vaccine-induced antibodies in mice
- PMID: 19901059
- PMCID: PMC2812197
- DOI: 10.1128/IAI.00877-09
Baculovirus-based nasal drop vaccine confers complete protection against malaria by natural boosting of vaccine-induced antibodies in mice
Abstract
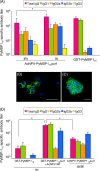
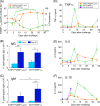
Blood-stage malaria parasites ablate memory B cells generated by vaccination in mice, resulting in diminishing natural boosting of vaccine-induced antibody responses to infection. Here we show the development of a new vaccine comprising a baculovirus-based Plasmodium yoelii 19-kDa carboxyl terminus of merozoite surface protein 1 (PyMSP1(19)) capable of circumventing the tactics of parasites in a murine model. The baculovirus-based vaccine displayed PyMSP1(19) on the surface of the virus envelope in its native three-dimensional structure. Needle-free intranasal immunization of mice with the baculovirus-based vaccine induced strong systemic humoral immune responses with high titers of PyMSP1(19)-specific antibodies. Most importantly, this vaccine conferred complete protection by natural boosting of vaccine-induced PyMSP1(19)-specific antibody responses shortly after challenge. The protective mechanism is a mixed Th1/Th2-type immunity, which is associated with the Toll-like receptor 9 (TLR9)-dependent pathway. The present study offers a novel strategy for the development of malaria blood-stage vaccines capable of naturally boosting vaccine-induced antibody responses to infection.
Figures



Similar articles
-
Protective immune responses elicited by immunization with a chimeric blood-stage malaria vaccine persist but are not boosted by Plasmodium yoelii challenge infection.Vaccine. 2010 Oct 4;28(42):6876-84. doi: 10.1016/j.vaccine.2010.08.018. Epub 2010 Aug 13. Vaccine. 2010. PMID: 20709001 Free PMC article.
-
Long-lasting protective immune response to the 19-kilodalton carboxy-terminal fragment of Plasmodium yoelii merozoite surface protein 1 in mice.Clin Vaccine Immunol. 2007 Apr;14(4):342-7. doi: 10.1128/CVI.00397-06. Epub 2007 Feb 21. Clin Vaccine Immunol. 2007. PMID: 17314232 Free PMC article.
-
A single injection of 19 kda carboxy-terminal fragment of Plasmodium yoelii merozoite surface protein 1 (PyMSP1(19)) formulated with Montanide ISA and CpG ODN induces protective immune response in mice.Asian Pac J Allergy Immunol. 2011 Sep;29(3):252-9. Asian Pac J Allergy Immunol. 2011. PMID: 22053595
-
Production, characterisation and immunogenicity of a plant-made Plasmodium antigen--the 19 kDa C-terminal fragment of Plasmodium yoelii merozoite surface protein 1.Appl Microbiol Biotechnol. 2012 Apr;94(1):151-61. doi: 10.1007/s00253-011-3772-7. Epub 2011 Dec 15. Appl Microbiol Biotechnol. 2012. PMID: 22170105
-
Immunity to erythrocytic stages of malarial parasites.Am J Trop Med Hyg. 1994;50(4 Suppl):27-32. doi: 10.4269/ajtmh.1994.50.27. Am J Trop Med Hyg. 1994. PMID: 7909653 Review.
Cited by
-
Characterization of the immune responses elicited by baculovirus-based vector vaccines against influenza virus hemagglutinin.Acta Pharmacol Sin. 2012 Jun;33(6):783-90. doi: 10.1038/aps.2012.23. Epub 2012 May 7. Acta Pharmacol Sin. 2012. PMID: 22562016 Free PMC article.
-
Baculovirus as a gene delivery vector: recent understandings of molecular alterations in transduced cells and latest applications.Biotechnol Adv. 2011 Nov-Dec;29(6):618-31. doi: 10.1016/j.biotechadv.2011.04.004. Epub 2011 Apr 28. Biotechnol Adv. 2011. PMID: 21550393 Free PMC article. Review.
-
Insufficiently defined genetic background confounds phenotypes in transgenic studies as exemplified by malaria infection in Tlr9 knockout mice.PLoS One. 2011;6(11):e27131. doi: 10.1371/journal.pone.0027131. Epub 2011 Nov 11. PLoS One. 2011. PMID: 22096530 Free PMC article.
-
Bombyx mori nucleopolyhedrovirus displaying neospora caninum antigens as a vaccine candidate against N. caninum infection in mice.Mol Biotechnol. 2015 Feb;57(2):145-54. doi: 10.1007/s12033-014-9810-9. Mol Biotechnol. 2015. PMID: 25307182
-
Plasmodium berghei circumvents immune responses induced by merozoite surface protein 1- and apical membrane antigen 1-based vaccines.PLoS One. 2010 Oct 28;5(10):e13727. doi: 10.1371/journal.pone.0013727. PLoS One. 2010. PMID: 21060850 Free PMC article.
References
-
- Abe, T., H. Takahashi, H. Hamazaki, N. Miyano-Kurosaki, Y. Matsuura, and H. Takaku. 2003. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J. Immunol. 171:1133-1139. - PubMed
-
- Coffman, R. L., B. W. Seymour, D. A. Lebman, D. D. Hiraki, J. A. Christiansen, B. Shrader, H. M. Cherwinski, H. F. Savelkoul, F. D. Finkelman, M. W. Bond, et al. 1988. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol. Rev. 102:5-28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

