CXCL10 production by human monocytes in response to Leishmania braziliensis infection
- PMID: 19901067
- PMCID: PMC2798186
- DOI: 10.1128/IAI.00959-09
CXCL10 production by human monocytes in response to Leishmania braziliensis infection
Abstract
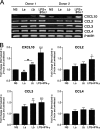
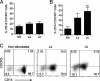
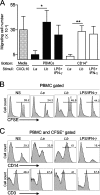
Leishmania (subgenus Viannia) braziliensis is the causative agent of mucocutaneous leishmaniasis (ML) in South America, and ML is characterized by excessive T- and B-cell responses to the parasite. We speculate that the unbalanced production of inflammatory mediators in response to L. braziliensis infection contributes to cell recruitment and disease severity. To test this hypothesis, we first examined the response of peripheral blood mononuclear cells (PBMCs) from healthy volunteers to L. braziliensis infection. We observed that while L. braziliensis infection induced the production of chemokine (C-X-C motif) ligand 10 (CXCL10) and interleukin-10 (IL-10) in human PBMCs and macrophages (MPhis), enhanced expression of CXCL10 and its receptor, chemokine CXC receptor (CXCR3), was predominantly detected in CD14(+) monocytes. The chemoattractant factors secreted by L. braziliensis-infected cells were highly efficient in recruiting uninfected PBMCs (predominantly CD14(+) cells) through Transwell membranes. Serum samples from American tegumentary leishmaniasis (ATL) patients (especially the ML cases) had significantly higher levels of CXCL10, CCL4, and soluble tumor necrosis factor (TNF) receptor II (sTNFRII) than did those of control subjects. Our results suggest that, following L. braziliensis infection, the production of multiple inflammatory mediators by the host may contribute to disease severity by increasing cellular recruitment.
Figures
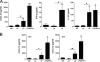


References
-
- Alexander, J., A. R. Satoskar, and D. G. Russell. 1999. Leishmania species: models of intracellular parasitism. J. Cell Sci. 112:2993-3002. - PubMed
-
- Amato, V. S., H. F. de Andrade, and M. I. Duarte. 2003. Mucosal leishmaniasis: in situ characterization of the host inflammatory response, before and after treatment. Acta Trop. 85:39-49. - PubMed
-
- Arevalo, J., L. Ramirez, V. Adaui, M. Zimic, G. Tulliano, C. Miranda-Verastegui, M. Lazo, R. Loayza-Muro, S. De Doncker, A. Maurer, F. Chappuis, J. C. Dujardin, and A. Llanos-Cuentas. 2007. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J. Infect. Dis. 195:1846-1851. - PubMed
-
- Asensio, V. C., J. Maier, R. Milner, K. Boztug, C. Kincaid, M. Moulard, C. Phillipson, K. Lindsley, T. Krucker, H. S. Fox, and I. L. Campbell. 2001. Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro. J. Virol. 75:7067-7077. - PMC - PubMed
-
- Auffray, C., M. H. Sieweke, and F. Geissmann. 2009. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 27:669-692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

