Aurora-A phosphorylates, activates, and relocalizes the small GTPase RalA
- PMID: 19901077
- PMCID: PMC2798468
- DOI: 10.1128/MCB.00916-08
Aurora-A phosphorylates, activates, and relocalizes the small GTPase RalA
Abstract
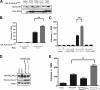
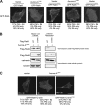
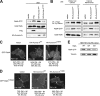
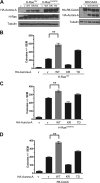
The small GTPase Ras, which transmits extracellular signals to the cell, and the kinase Aurora-A, which promotes proper mitosis, can both be inappropriately activated in human tumors. Here, we show that Aurora-A in conjunction with oncogenic Ras enhances transformed cell growth. Furthermore, such transformation and in some cases also tumorigenesis depend upon S194 of RalA, a known Aurora-A phosphorylation site. Aurora-A promotes not only RalA activation but also translocation from the plasma membrane and activation of the effector protein RalBP1. Taken together, these data suggest that Aurora-A may converge upon oncogenic Ras signaling through RalA.
Figures





References
-
- Anand, S., S. Penrhyn-Lowe, and A. R. Venkitaraman. 2003. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell 3:51-62. - PubMed
-
- Awasthi, S., J. Cheng, S. S. Singhal, M. K. Saini, U. Pandya, S. Pikula, J. Bandorowicz-Pikula, S. V. Singh, P. Zimniak, and Y. C. Awasthi. 2000. Novel function of human RLIP76: ATP-dependent transport of glutathione conjugates and doxorubicin. Biochemistry 39:9327-9334. - PubMed
-
- Awasthi, S., S. S. Singhal, S. Yadav, J. Singhal, K. Drake, A. Nadkar, E. Zajac, D. Wickramarachchi, N. Rowe, A. Yacoub, P. Boor, S. Dwivedi, P. Dent, W. E. Jarman, B. John, and Y. C. Awasthi. 2005. RLIP76 is a major determinant of radiation sensitivity. Cancer Res. 65:6022-6028. - PubMed
-
- Bagrodia, S., S. J. Taylor, C. L. Creasy, J. Chernoff, and R. A. Cerione. 1995. Identification of a mouse p21Cdc42/Rac activated kinase. J. Biol. Chem. 270:22731-22737. - PubMed
-
- Bischoff, J. R., L. Anderson, Y. Zhu, K. Mossie, L. Ng, B. Souza, B. Schryver, P. Flanagan, F. Clairvoyant, C. Ginther, C. S. Chan, M. Novotny, D. J. Slamon, and G. D. Plowman. 1998. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 17:3052-3065. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
