A novel class of meso-tetrakis-porphyrin derivatives exhibits potent activities against hepatitis C virus genotype 1b replicons in vitro
- PMID: 19901090
- PMCID: PMC2798527
- DOI: 10.1128/AAC.01206-09
A novel class of meso-tetrakis-porphyrin derivatives exhibits potent activities against hepatitis C virus genotype 1b replicons in vitro
Abstract
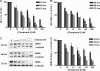
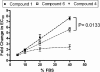
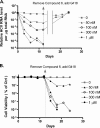
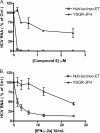
Recent years have seen the rapid advancement of new therapeutic agents against hepatitis C virus (HCV) in response to the need for treatment that is unmet by interferon (IFN)-based therapies. Most antiviral drugs discovered to date are small molecules that modulate viral enzyme activities. In the search for highly selective protein-binding molecules capable of disrupting the viral life cycle, we have identified a class of anionic tetraphenylporphyrins as potent and specific inhibitors of the HCV replicons. Based on the structure-activity relationship studies reported herein, meso-tetrakis-(3,5-dicarboxy-4,4'-biphenyl) porphyrin was found to be the most potent inhibitor of HCV genotype 1b (Con1) replicon systems but was less effective against the genotype 2a (JFH-1) replicon. This compound induced a reduction of viral RNA and protein levels when acting in the low nanomolar range. Moreover, the compound could suppress replicon rebound in drug-treated cells and exhibited additive to synergistic effects when combined with protease inhibitor BILN 2061 or with IFN-alpha-2a. Our results demonstrate the potential use of tetracarboxyphenylporphyrins as potent anti-HCV agents.
Figures

References
-
- Aya, T., and A. D. Hamilton. 2003. Tetrabiphenylporphyrin-based receptors for protein surfaces show sub-nanomolar affinity and enhance unfolding. Bioorg. Med. Chem. Lett. 13:2651-2654. - PubMed
-
- Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1974. - PubMed
-
- Chou, T.-C. 1991. The median-effect principle and the combination index for quantitation of synergism and antagonism, p. 61-102. In D. C. Rideout and T.-C. Chou (ed.), Synergism and antagonism in chemotherapy. Academic Press, Inc., San Diego, CA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

