Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects
- PMID: 19901119
- PMCID: PMC2793040
- DOI: 10.1200/JCO.2008.20.4156
Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects
Abstract
Purpose: Methotrexate plasma concentration is related to its clinical effects. Our aim was to identify the genetic basis of interindividual variability in methotrexate pharmacokinetics in children with newly diagnosed acute lymphoblastic leukemia (ALL).
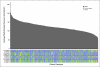
Patients and methods: We performed a genome-wide analysis of 500,568 germline single-nucleotide polymorphisms (SNPs) to identify how inheritance affects methotrexate plasma disposition among 434 children with ALL who received 3,014 courses of methotrexate at 2 to 5 g/m(2). SNPs were validated in an independent cohort of 206 patients.
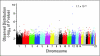
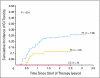
Results: Adjusting for age, race, sex, and methotrexate regimen, the most significant associations were with SNPs in the organic anion transporter polypeptide, SLCO1B1. Two SNPs in SLCO1B1, rs11045879 (P = 1.7 x 10(-10)) and rs4149081 (P = 1.7 x 10(-9)), were in linkage disequilibrium (LD) with each other (r(2) = 1) and with a functional polymorphism in SLCO1B1, T521C (rs4149056; r(2) > 0.84). rs11045879 and rs4149081 were validated in an independent cohort of 206 patients (P = .018 and P = .017), as were other SLCO1B1 SNPs residing in different LD blocks. SNPs in SLCO1B1 were also associated with GI toxicity (odds ratio, 15.3 to 16.4; P = .03 to .004).
Conclusion: A genome-wide interrogation identified inherited variations in a plausible, yet heretofore low-priority candidate gene, SLCO1B1, as important determinants of methotrexate's pharmacokinetics and clinical effects.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Similar articles
-
Germline genetic variations in methotrexate candidate genes are associated with pharmacokinetics, toxicity, and outcome in childhood acute lymphoblastic leukemia.Blood. 2013 Jun 27;121(26):5145-53. doi: 10.1182/blood-2013-01-480335. Epub 2013 May 7. Blood. 2013. PMID: 23652803
-
Rare versus common variants in pharmacogenetics: SLCO1B1 variation and methotrexate disposition.Genome Res. 2012 Jan;22(1):1-8. doi: 10.1101/gr.129668.111. Epub 2011 Dec 6. Genome Res. 2012. PMID: 22147369 Free PMC article.
-
Polymorphisms of the SLCO1B1 gene predict methotrexate-related toxicity in childhood acute lymphoblastic leukemia.Pediatr Blood Cancer. 2011 Oct;57(4):612-9. doi: 10.1002/pbc.23074. Epub 2011 Mar 8. Pediatr Blood Cancer. 2011. PMID: 21387541
-
Methotrexate Disposition in Pediatric Patients with Acute Lymphoblastic Leukemia: What Have We Learnt From the Genetic Variants of Drug Transporters.Curr Pharm Des. 2019;25(6):627-634. doi: 10.2174/1381612825666190329141003. Curr Pharm Des. 2019. PMID: 30931851 Review.
-
Systematic review: genetic polymorphisms in the pharmacokinetics of high-dose methotrexate in pediatric acute lymphoblastic leukemia patients.Cancer Chemother Pharmacol. 2024 Aug;94(2):141-155. doi: 10.1007/s00280-024-04694-0. Epub 2024 Jul 13. Cancer Chemother Pharmacol. 2024. PMID: 39002021
Cited by
-
Bringing genome-wide association findings into clinical use.Nat Rev Genet. 2013 Aug;14(8):549-58. doi: 10.1038/nrg3523. Epub 2013 Jul 9. Nat Rev Genet. 2013. PMID: 23835440 Review.
-
CYP2C9 and OATP1B1 genetic polymorphisms affect the metabolism and transport of glimepiride and gliclazide.Sci Rep. 2018 Jul 20;8(1):10994. doi: 10.1038/s41598-018-29351-4. Sci Rep. 2018. PMID: 30030468 Free PMC article.
-
Polymorphisms in folate pathway and pemetrexed treatment outcome in patients with malignant pleural mesothelioma.Radiol Oncol. 2014 Apr 25;48(2):163-72. doi: 10.2478/raon-2013-0086. eCollection 2014 Jun. Radiol Oncol. 2014. PMID: 24991206 Free PMC article.
-
Genome-Wide Association Studies of Chemotherapeutic Toxicities: Genomics of Inequality.Clin Cancer Res. 2017 Aug 1;23(15):4010-4019. doi: 10.1158/1078-0432.CCR-17-0429. Epub 2017 Apr 25. Clin Cancer Res. 2017. PMID: 28442506 Free PMC article. Review.
-
Complete OATP1B1 and OATP1B3 deficiency causes human Rotor syndrome by interrupting conjugated bilirubin reuptake into the liver.J Clin Invest. 2012 Feb;122(2):519-28. doi: 10.1172/JCI59526. Epub 2012 Jan 9. J Clin Invest. 2012. PMID: 22232210 Free PMC article.
References
-
- Gudmundsson J, Sulem P, Steinthorsdottir V, et al. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet. 2007;39:977–983. - PubMed
-
- Hakonarson H, Grant SF, Bradfield JP, et al. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature. 2007;448:591–594. - PubMed
-
- Yeager M, Orr N, Hayes RB, et al. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet. 2007;39:645–649. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

