Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase
- PMID: 19901187
- PMCID: PMC2787902
- DOI: 10.1161/CIRCULATIONAHA.109.884403
Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase
Abstract
Background: Despite numerous studies demonstrating the efficacy of cellular adoptive transfer for therapeutic myocardial regeneration, problems remain for donated cells with regard to survival, persistence, engraftment, and long-term benefits. This study redresses these concerns by enhancing the regenerative potential of adoptively transferred cardiac progenitor cells (CPCs) via genetic engineering to overexpress Pim-1, a cardioprotective kinase that enhances cell survival and proliferation.
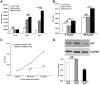
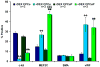
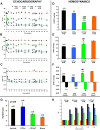
Methods and results: Intramyocardial injections of CPCs overexpressing Pim-1 were given to infarcted female mice. Animals were monitored over 4, 12, and 32 weeks to assess cardiac function and engraftment of Pim-1 CPCs with echocardiography, in vivo hemodynamics, and confocal imagery. CPCs overexpressing Pim-1 showed increased proliferation and expression of markers consistent with cardiogenic lineage commitment after dexamethasone exposure in vitro. Animals that received CPCs overexpressing Pim-1 also produced greater levels of cellular engraftment, persistence, and functional improvement relative to control CPCs up to 32 weeks after delivery. Salutary effects include reduction of infarct size, greater number of c-kit(+) cells, and increased vasculature in the damaged region.
Conclusions: Myocardial repair is significantly enhanced by genetic engineering of CPCs with Pim-1 kinase. Ex vivo gene delivery to enhance cellular survival, proliferation, and regeneration may overcome current limitations of stem cell-based therapeutic approaches.
Conflict of interest statement
Figures




References
-
- Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102:8966–8971. - PMC - PubMed
-
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. - PubMed
-
- Leri A, Kajstura J, Anversa P, Frishman WH. Myocardial regeneration and stem cell repair. Curr Probl Cardiol. 2008;33:91–153. - PubMed
-
- Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Muller P, Esposito G, Bearzi C, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, Leri A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

