Multiple myeloma phosphotyrosine proteomic profile associated with FGFR3 expression, ligand activation, and drug inhibition
- PMID: 19901323
- PMCID: PMC2775037
- DOI: 10.1073/pnas.0910957106
Multiple myeloma phosphotyrosine proteomic profile associated with FGFR3 expression, ligand activation, and drug inhibition
Abstract
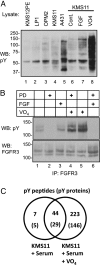
Signaling by growth factor receptor tyrosine kinases is manifest through networks of proteins that are substrates and/or bind to the activated receptors. FGF receptor-3 (FGFR3) is a drug target in a subset of human multiple myelomas (MM) and is mutationally activated in some cervical and colon and many bladder cancers and in certain skeletal dysplasias. To define the FGFR3 network in multiple myeloma, mass spectrometry was used to identify and quantify phosphotyrosine (pY) sites modulated by FGFR3 activation and inhibition in myeloma-derived KMS11 cells. Label-free quantification of peptide ion currents indicated the activation of FGFR3 by phosphorylation of tandem tyrosines in the kinase domain activation loop when cellular pY phosphatases were inhibited by pervanadate. Among the 175 proteins that accumulated pY in response to pervanadate was a subset of 52 including FGFR3 that contained a total of 61 pY sites that were sensitive to inhibition by the FGFR3 inhibitor PD173074. The FGFR3 isoform containing the tandem pY motif in its activation loop was targeted by PD173074. Forty of the drug-sensitive pY sites, including two located within the 35-residue cytoplasmic domain of the transmembrane growth factor binding proteoglycan (and multiple myeloma biomarker) Syndecan-1/CD138, were also stimulated in cells treated with the ligand FGF1, providing additional validation of their link to FGFR3. The identification of these overlapping sets of co-modulated tyrosine phosphorylations presents an outline of an FGFR3 network in the MM model and demonstrates the potential for pharmacodynamic monitoring by label-free quantitative phospho-proteomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Blume-Jensen P, Hunter T. Oncogenic kinase signaling. Nature. 2001;411:355–365. - PubMed
-
- Paterson JL, et al. Preclinical studies of fibroblast growth factor receptor 3 as a therapeutic target in multiple myeloma. Br J Haematol. 2004;124:595–603. - PubMed
-
- Hideshima T, Bergsagel PL, Kuehl WM, Anderson KC. Advances in biology of multiple myeloma: Clinical applications. Blood. 2004;104:607–618. - PubMed
-
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. - PubMed
-
- Furdui CM, Lew ED, Schlessinger J, Anderson KS. Autophosphorylation of FGFR1 kinase is mediated by a sequential and precisely ordered reaction. Mol Cell. 2006;21:711–717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

