Simple cellular and network control principles govern complex patterns of motor behavior
- PMID: 19901329
- PMCID: PMC2785286
- DOI: 10.1073/pnas.0906722106
Simple cellular and network control principles govern complex patterns of motor behavior
Abstract
The vertebrate central nervous system is organized in modules that independently execute sophisticated tasks. Such modules are flexibly controlled and operate with a considerable degree of autonomy. One example is locomotion generated by spinal central pattern generator networks (CPGs) that shape the detailed motor output. The level of activity is controlled from brainstem locomotor command centers, which in turn, are under the control of the basal ganglia. By using a biophysically detailed, full-scale computational model of the lamprey CPG (10,000 neurons) and its brainstem/forebrain control, we demonstrate general control principles that can adapt the network to different demands. Forward or backward locomotion and steering can be flexibly controlled by local synaptic effects limited to only the very rostral part of the network. Variability in response properties within each neuronal population is an essential feature and assures a constant phase delay along the cord for different locomotor speeds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
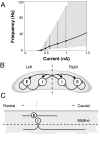




References
-
- Grillner S, Hellgren J, Ménard A, Saitoh K, Wikström MA. Mechanisms for selection of basic motor programs–roles for the striatum and pallidum. Trends Neurosci. 2005;28:364–370. - PubMed
-
- Hikosaka O. GABAergic output of the basal ganglia. Prog Brain Res. 2007;160:209–226. - PubMed
-
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. - PubMed
-
- Roberts A, Li WC, Soffe SR, Wolf E. Origin of excitatory drive to a spinal locomotor network. Brain Res Rev. 2008;57:22–28. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

