Plasmacytoid dendritic cells prevent cigarette smoke and Chlamydophila pneumoniae-induced Th2 inflammatory responses
- PMID: 19901344
- PMCID: PMC2951872
- DOI: 10.1165/rcmb.2009-0224OC
Plasmacytoid dendritic cells prevent cigarette smoke and Chlamydophila pneumoniae-induced Th2 inflammatory responses
Abstract
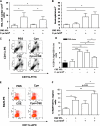
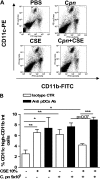
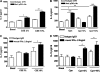
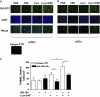
Smoking promotes the development of allergic asthma and pneumonia. Chlamydophila pneumoniae lung infection is associated with an increased risk for asthma, inducing an immune response regulated by dendritic cells (DCs). This study sought to determine whether exposure to cigarette smoke modulates the functional activity of CD11c-positive DCs in the lung, with and without concomitant C. pneumoniae infection. Bone marrow-derived DCs (BMDCs) were exposed in vitro to cigarette smoke extract (CSE) and/or live C. pneumoniae (Cpn), and then adoptively transferred intratracheally into wild-type mice. Although CSE plus Cpn appeared to exert an additive effect on the production of Th2 cytokines in vitro, we did not see this effect in vivo. However, the adoptive transfer of DCs pulsed with both CSE and C. pneumoniae into the lungs of naive mice led to an influx of plasmacytoid DCs (pDCs) that suppressed the Th2 skewing ability of the transferred BMDCs. The depletion of pDCs by antibody restored the Th2 skewing ability of the BMDCs. The expression of indoleamine-2,3-dioxygenase in the lung was reduced after the depletion of pDCs, and blocking IFN-α in vitro prevented the ability of pDCs to inhibit the Th2 responses induced by myeloid DCs (mDCs), suggesting their potential involvement in the mechanism of altered polarization. In conclusion, exposure to cigarette smoke skews C. pneumoniae-induced mDCs responses toward a Th2 bias in the lung, which is prevented by pDCs. We propose that pDCs may play a major role in the immunosuppressive lung environment in smokers with C. pneumoniae infection.
Figures



References
-
- Chalmers GW, MacLeod KJ, Thomson L, Little SA, McSharry C, Thomson NC. Smoking and airway inflammation in patients with mild asthma. Chest 2001;120:1917–1922. - PubMed
-
- Hylkema MN, Sterk PJ, de Boer WI, Postma DS. Tobacco use in relation to COPD and asthma. Eur Respir J 2007;29:438–445. - PubMed
-
- Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release MIP-3α/CCL-20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol 2003;28:648–654. - PubMed
-
- Naiki Y, Sorrentino R, Wong MH, Michelsen KS, Shimada K, Chen S, Yilmaz A, Slepenkin A, Schröder NW, Crother TR, et al. TLR/MyD88 and liver X receptor alpha signaling pathways reciprocally control Chlamydia pneumoniae-induced acceleration of atherosclerosis. J Immunol 2008;181:7176–7185. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

