Neurotransmitters in airway parasympathetic neurons altered by neurotrophin-3 and repeated allergen challenge
- PMID: 19901346
- PMCID: PMC2951875
- DOI: 10.1165/rcmb.2009-0130OC
Neurotransmitters in airway parasympathetic neurons altered by neurotrophin-3 and repeated allergen challenge
Abstract
Changes in airway nerves associated with chronic inflammation may underlie the pathogenesis and symptoms of lower airway diseases, such as asthma. The molecules most likely causing such alterations are neurotrophins (NTs) and/or related neurokines. In several species, including humans, lower airway parasympathetic postganglionic neurons that project axons to airway smooth muscle are either cholinergic or nonadrenergic noncholinergic (NANC), the latter synthesizing vasoactive intestinal peptide and nitric oxide, but not acetylcholine. In guinea pig trachealis smooth muscle, cholinergic nerve terminals arise from ganglionic neurons located near the tracheal smooth muscle, whereas the source of NANC nerve fibers is from neurons in ganglia located in the adjacent myenteric plexus of the esophagus, making this an ideal species to study regulation of parasympathetic neurotransmitter phenotypes. In the present study, we determined that, 48 hours after repeated allergen challenge, the NANC phenotype of airway parasympathetic ganglionic neurons changed to a cholinergic phenotype, and NT-3 mimicked this change. Nerve growth factor, brain-derived neurotrophic factor, leukemia inhibitory factor, or IL-1β had no effect on either phenotype, and they did not induce these neurons to synthesize substance P or tyrosine hydroxylase. These results indicate a role for inflammation and NT-3 in regulating biochemical and anatomical characteristics of principal neurons in adult airway parasympathetic ganglia.
Figures
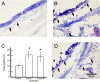

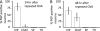

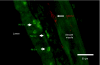
Similar articles
-
Neurotransmitters in parasympathetic ganglionic neurons and nerves in mouse lower airway smooth muscle.Respir Physiol Neurobiol. 2013 Oct 1;189(1):195-202. doi: 10.1016/j.resp.2013.07.006. Epub 2013 Jul 24. Respir Physiol Neurobiol. 2013. PMID: 23891709
-
Effects of organotypic culture on parasympathetic innervation of guinea pig trachealis.Am J Physiol. 1996 Nov;271(5 Pt 1):L698-706. doi: 10.1152/ajplung.1996.271.5.L698. Am J Physiol. 1996. PMID: 8944712
-
Evidence for an esophageal origin of VIP-IR and NO synthase-IR nerves innervating the guinea pig trachealis: a retrograde neuronal tracing and immunohistochemical analysis.J Comp Neurol. 1998 May 11;394(3):326-34. J Comp Neurol. 1998. PMID: 9579396
-
Autonomic innervation of human airways: structure, function, and pathophysiology in asthma.Neuroimmunomodulation. 1999 May-Jun;6(3):145-59. doi: 10.1159/000026376. Neuroimmunomodulation. 1999. PMID: 10213912 Review.
-
Innervation of airway smooth muscle. Efferent mechanisms.Pharmacol Ther. 1987;32(2):107-30. doi: 10.1016/0163-7258(87)90055-6. Pharmacol Ther. 1987. PMID: 2885861 Review.
Cited by
-
Neurotrophins in Asthma.Curr Allergy Asthma Rep. 2018 Feb 16;18(2):10. doi: 10.1007/s11882-018-0765-y. Curr Allergy Asthma Rep. 2018. PMID: 29453651 Free PMC article. Review.
-
Eosinophils increase airway sensory nerve density in mice and in human asthma.Sci Transl Med. 2018 Sep 5;10(457):eaar8477. doi: 10.1126/scitranslmed.aar8477. Sci Transl Med. 2018. PMID: 30185653 Free PMC article.
-
Airway Innervation and Plasticity in Asthma.Physiology (Bethesda). 2019 Jul 1;34(4):283-298. doi: 10.1152/physiol.00050.2018. Physiology (Bethesda). 2019. PMID: 31165683 Free PMC article. Review.
-
Uterine Inflammation Changes the Expression of Cholinergic Neurotransmitters and Decreases the Population of AChE-Positive, Uterus-Innervating Neurons in the Paracervical Ganglion of Sexually Mature Gilts.Animals (Basel). 2022 Jun 29;12(13):1676. doi: 10.3390/ani12131676. Animals (Basel). 2022. PMID: 35804576 Free PMC article.
-
The mode of action of anticholinergics in asthma.Eur Respir J. 2018 Oct 4;52(4):1701247. doi: 10.1183/13993003.01247-2017. Print 2018 Oct. Eur Respir J. 2018. PMID: 30115613 Free PMC article. Review.
References
-
- Nockher WA, Renz H. Neurotrophins and asthma: novel insight into neuroimmune interaction. J Allergy Clin Immunol 2006;117:67–71. - PubMed
-
- Jacoby DB, Fryer AD. Anticholinergic therapy for airway diseases. Life Sci 2001;68:2565–2572. - PubMed
-
- Carr MJ, Hunter DD, Undem BJ. Neurotrophins and asthma. Curr Opin Pulm Med 2001;7:1–7. - PubMed
-
- Virchow JC, Julius P, Lommatzsch M, Luttman W, Renz H, Braun A. Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am J Respir Crit Care Med 1998;58:2002–2005. - PubMed
-
- Tortorolo L, Langer A, Polidori G, Vento G, Stampachiacchere B, Aloe L, Piedimonte G. Neurotrophin overexpression in lower airways of infants with respiratory syncytial virus infection. Am J Respir Crit Care Med 2005;172:233–237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

