A novel N-terminal motif of dipeptidyl peptidase-like proteins produces rapid inactivation of KV4.2 channels by a pore-blocking mechanism
- PMID: 19901547
- PMCID: PMC3607289
- DOI: 10.4161/chan.3.6.10216
A novel N-terminal motif of dipeptidyl peptidase-like proteins produces rapid inactivation of KV4.2 channels by a pore-blocking mechanism
Abstract
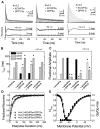
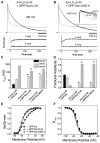
The somatodendritic subthreshold A-type K(+) current in neurons (I(SA)) depends on its kinetic and voltage-dependent properties to regulate membrane excitability, action potential repetitive firing, and signal integration. Key functional properties of the K(V)4 channel complex underlying I(SA) are determined by dipeptidyl peptidase-like proteins known as dipeptidyl peptidase 6 (DPP6) and dipeptidyl peptidase 10 (DPP10). Among the multiple known DPP10 isoforms with alternative N-terminal sequences, DPP10a confers exceptionally fast inactivation to K(V)4.2 channels. To elucidate the molecular basis of this fast inactivation, we investigated the structure-function relationship of the DPP10a N-terminal region and its interaction with the K(V)4.2 channel. Here, we show that DPP10a shares a conserved N-terminal sequence (MNQTA) with DPP6a (aka DPP6-E), which also induces fast inactivation. Deletion of the NQTA sequence in DPP10a eliminates this dramatic fast inactivation, and perfusion of MNQTA peptide to the cytoplasmic face of inside-out patches inhibits the K(V)4.2 current. DPP10a-induced fast inactivation exhibits competitive interactions with internally applied tetraethylammonium (TEA), and elevating the external K(+) concentration accelerates recovery from DPP10a-mediated fast inactivation. These results suggest that fast inactivation induced by DPP10a or DPP6a is mediated by a common N-terminal inactivation motif via a pore-blocking mechanism. This mechanism may offer an attractive target for novel pharmacological interventions directed at impairing I(SA) inactivation and reducing neuronal excitability.
Figures

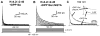






References
-
- Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004;27:343–369. - PubMed
-
- Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev. 2004;84:803–833. - PubMed
-
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
