Pancreatic duodenal homeobox 1 protein is a novel beta-cell-specific autoantigen for type I diabetes
- PMID: 19901909
- PMCID: PMC3408089
- DOI: 10.1038/labinvest.2009.116
Pancreatic duodenal homeobox 1 protein is a novel beta-cell-specific autoantigen for type I diabetes
Abstract
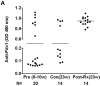
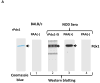
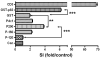
Pancreatic duodenal homeobox 1 (Pdx1) protein is a key transcription factor involved in the regulation of insulin gene expression that is expressed at high levels in the beta-cells of the pancreatic islets. We asked whether Pdx1 is a target of anti-islet autoimmunity in type I diabetes (T1D). Pdx1 autoantibodies (PAAs) were detected in non-obese diabetic (NOD) mice using ELISA, western blotting, and radioimmunoprecipitation of [(35)S]-labeled insulinoma cell line-derived Pdx1 protein. PAAs were detected as early as at 5 weeks of age, and generally peaked before the onset of clinically overt diabetes in diabetes-prone female NOD mice. Levels declined substantially after the onset of diabetes. PAAs were not detected in the sera of NOD-scid, C57BL/6, or BALB/c mice. The titers of PAAs in NOD mouse sera were as high as 1/93 750 by ELISA. The fine specificity of PAAs was determined by western blotting using a series of truncated recombinant Pdx1 proteins. The immunodominant epitopes were located to the C-terminus of the Pdx1 (p200-283) in NOD mice. PAAs also were detected in sera from human T1D patients, but the major epitopes were localized to amino acids 159-200 as well as the same region (p200-283) recognized by PAAs from NOD mice. Using [(3)H]thymidine incorporation, the p83 fragment of Pdx1 specifically stimulated proliferation of splenic T cells from recent-onset diabetic NOD mice. The presence of PAAs in prediabetic NOD mice and human T1D patients, and Pdx1-specific T-cell proliferation in NOD mice provide a strong rationale for further investigation of the pathogenic role of immune responses against Pdx1 in T1D.
Figures









Comment in
-
Attacking the source: anti-PDX-1 responses in type 1 diabetes.Lab Invest. 2010 Jan;90(1):6-8. doi: 10.1038/labinvest.2009.121. Lab Invest. 2010. PMID: 20038945 Free PMC article.
References
-
- Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. - PubMed
-
- Wasserfall CH, Atkinson MA. Autoantibody markers for the diagnosis and prediction of type 1 diabetes. Autoimmun Rev. 2006;5:424–428. - PubMed
-
- Miao D, Yu L, Eisenbarth GS. Role of autoantibodies in type 1 diabetes. Front Biosci. 2007;12:1889–1898. - PubMed
-
- Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54(Suppl 2):S52–S61. - PubMed
-
- Skyler JS. Prediction and prevention of type 1 diabetes: progress, problems, and prospects. Clin Pharmacol Ther. 2007;81:768–771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

