Trial publication after registration in ClinicalTrials.Gov: a cross-sectional analysis
- PMID: 19901971
- PMCID: PMC2728480
- DOI: 10.1371/journal.pmed.1000144
Trial publication after registration in ClinicalTrials.Gov: a cross-sectional analysis
Abstract
Background: ClinicalTrials.gov is a publicly accessible, Internet-based registry of clinical trials managed by the US National Library of Medicine that has the potential to address selective trial publication. Our objectives were to examine completeness of registration within ClinicalTrials.gov and to determine the extent and correlates of selective publication.
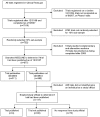
Methods and findings: We examined reporting of registration information among a cross-section of trials that had been registered at ClinicalTrials.gov after December 31, 1999 and updated as having been completed by June 8, 2007, excluding phase I trials. We then determined publication status among a random 10% subsample by searching MEDLINE using a systematic protocol, after excluding trials completed after December 31, 2005 to allow at least 2 y for publication following completion. Among the full sample of completed trials (n = 7,515), nearly 100% reported all data elements mandated by ClinicalTrials.gov, such as intervention and sponsorship. Optional data element reporting varied, with 53% reporting trial end date, 66% reporting primary outcome, and 87% reporting trial start date. Among the 10% subsample, less than half (311 of 677, 46%) of trials were published, among which 96 (31%) provided a citation within ClinicalTrials.gov of a publication describing trial results. Trials primarily sponsored by industry (40%, 144 of 357) were less likely to be published when compared with nonindustry/nongovernment sponsored trials (56%, 110 of 198; p<0.001), but there was no significant difference when compared with government sponsored trials (47%, 57 of 122; p = 0.22). Among trials that reported an end date, 75 of 123 (61%) completed prior to 2004, 50 of 96 (52%) completed during 2004, and 62 of 149 (42%) completed during 2005 were published (p = 0.006).
Conclusions: Reporting of optional data elements varied and publication rates among completed trials registered within ClinicalTrials.gov were low. Without greater attention to reporting of all data elements, the potential for ClinicalTrials.gov to address selective publication of clinical trials will be limited. Please see later in the article for the Editors' Summary.
Conflict of interest statement
JSR, GKM, and HMK were previously consultants at the request of plaintiffs in litigation against Merck and Co., related to rofecoxib. SEN has received research support to perform clinical trials through the Cleveland Clinic Coordinating Center for Clinical Research from Pfizer, Astra Zeneca, Daiichi-Sankyo, Takeda, Roche, Novartis, Sanofi-Aventis, and Eli Lilly. SEN consults for Novartis, Anylam, Sanofi-Aventis, Astra Zeneca, Forbes Meditech, Ganedon, Genzyme, Hollis-Eden, Eli Lilly, Pfizer, Karo Bio, Novo Nordisk, Roche, Daiichi-Sankyo, Takeda, Resverlogix, and Glaxo-Smith-Kline, but requires them to donate all honoraria or consulting fees directly to charity so that he receives neither income nor a tax deduction. HMK has research contracts with the American College of Cardiology and the Colorado Foundation for Medical Care, has served on the advisory boards of Amgen and UnitedHealthcare, has been a subject expert for VHA, and is academic_editor-in-Chief of
Figures
References
-
- Lee K, Bacchetti P, Sim I. Publication of clinical trials supporting successful new drug applications: a literature analysis. PLoS Med. 2008;5:e191. doi: 10.1371/journal.pmed.0050191. - DOI - PMC - PubMed
-
- Rising K, Bacchetti P, Bero LA. Reporting bias in drug trials submitted to the Food and Drug Administration: review of publication and presentation. PLoS Med. 2008;5:e217. doi: 10.1371/journal.pmed.0050217. - DOI - PMC - PubMed
-
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. - PubMed
-
- Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299:914–924. - PubMed
-
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252–260. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical