Phenylalanine-rich peptides potently bind ESAT6, a virulence determinant of Mycobacterium tuberculosis, and concurrently affect the pathogen's growth
- PMID: 19901982
- PMCID: PMC2768790
- DOI: 10.1371/journal.pone.0007615
Phenylalanine-rich peptides potently bind ESAT6, a virulence determinant of Mycobacterium tuberculosis, and concurrently affect the pathogen's growth
Abstract
Background: The secretory proteins of Mycobacterium tuberculosis (M. tuberculosis) have been known to be involved in the virulence, pathogenesis as well as proliferation of the pathogen. Among this set, many proteins have been hypothesized to play a critical role at the genesis of the onset of infection, the primary site of which is invariably the human lung.
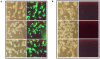
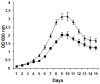
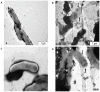
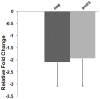
Methodology/principal findings: During our efforts to isolate potential binding partners of key secretory proteins of M. tuberculosis from a human lung protein library, we isolated peptides that strongly bound the virulence determinant protein Esat6. All peptides were less than fifty amino acids in length and the binding was confirmed by in vivo as well as in vitro studies. Curiously, we found all three binders to be unusually rich in phenylalanine, with one of the three peptides a short fragment of the human cytochrome c oxidase-3 (Cox-3). The most accessible of the three binders, named Hcl1, was shown also to bind to the Mycobacterium smegmatis (M. smegmatis) Esat6 homologue. Expression of hcl1 in M. tuberculosis H37Rv led to considerable reduction in growth. Microarray analysis showed that Hcl1 affects a host of key cellular pathways in M. tuberculosis. In a macrophage infection model, the sets expressing hcl1 were shown to clear off M. tuberculosis in much greater numbers than those infected macrophages wherein the M. tuberculosis was not expressing the peptide. Transmission electron microscopy studies of hcl1 expressing M. tuberculosis showed prominent expulsion of cellular material into the matrix, hinting at cell wall damage.
Conclusions/significance: While the debilitating effects of Hcl1 on M. tuberculosis are unrelated and not because of the peptide's binding to Esat6-as the latter is not an essential protein of M. tuberculosis-nonetheless, further studies with this peptide, as well as a closer inspection of the microarray data may shed important light on the suitability of such small phenylalanine-rich peptides as potential drug-like molecules against this pathogen.
Conflict of interest statement
Figures



References
-
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. J Am Med Assoc. 1999;282:677–686. - PubMed
-
- Yew WW, Leung CC. Updates in tuberculosis 2007. Am J Respir Crit Care Med. 2008;177:479–485. - PubMed
-
- Khatri GR. Controlling tuberculosis in India. New Engl J Med. 2002;347:1420–1425. - PubMed
-
- Jain A, Mondal R. Extensively drug-resistant tuberculosis: current challenges and threats. FEMS Immunol Med Microbiol. 2008;53:145–150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

