Quantitative proteomics analysis of maternal plasma in Down syndrome pregnancies using isobaric tagging reagent (iTRAQ)
- PMID: 19902006
- PMCID: PMC2774473
- DOI: 10.1155/2010/952047
Quantitative proteomics analysis of maternal plasma in Down syndrome pregnancies using isobaric tagging reagent (iTRAQ)
Abstract
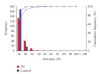
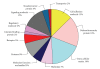
Currently no specific biomarkers exist for the screening of pregnancies at risk for Down syndrome (DS). Since a quantitative proteomic approach with isobaric labelling (iTRAQ) has recently been suggested to be highly suitable for the discovery of novel plasma biomarkers, we have now used this method to examine for potential quantitative changes in the plasma proteome of the pregnancies bearing DS fetuses in comparison to normal healthy babies. In our study, we used plasma from six women with DS pregnancies and six with uncomplicated pregnancies care were taken to match cases and controls for gestational and maternal age, as these could be a confounder. In our quantitative proteomics analysis we were able to detect 178 proteins using iTRAQ labelling in conjunction with 4800 MALDI TOF/TOF. Amongst these we observed changes in betaHCG, a known screening marker for DS, indicating that our assay was functional. We found a number of elevated proteins Ig lambda chain C region, serum amyloid P-component, amyloid beta A4, and under expressed proteins like gamma-actin and titin in DS pregnancies. These proteins are also found in the sera of patients with Alzheimer disease, which share similar pathologies of DS. Our study therefore indicates that the iTRAQ labelling approach may be indeed useful for the detection of novel biomarkers.
Figures



Similar articles
-
Quantitative proteomic (iTRAQ) analysis of 1st trimester maternal plasma samples in pregnancies at risk for preeclampsia.J Biomed Biotechnol. 2012;2012:305964. doi: 10.1155/2012/305964. Epub 2012 Apr 10. J Biomed Biotechnol. 2012. PMID: 22570525 Free PMC article.
-
Quantitative proteomic analysis of down syndrome biomarkers in maternal serum using isobaric tags for relative and absolute quantification (iTRAQ).Gynecol Endocrinol. 2020 Jun;36(6):489-495. doi: 10.1080/09513590.2019.1696302. Epub 2019 Dec 3. Gynecol Endocrinol. 2020. PMID: 31793358
-
Preliminary proteomic-based identification of a novel protein for Down's syndrome in maternal serum.Exp Biol Med (Maywood). 2012 May;237(5):530-9. doi: 10.1258/ebm.2012.011312. Exp Biol Med (Maywood). 2012. PMID: 22678011
-
Isobaric protein and peptide quantification: perspectives and issues.Expert Rev Proteomics. 2010 Oct;7(5):647-53. doi: 10.1586/epr.10.29. Expert Rev Proteomics. 2010. PMID: 20973638 Review.
-
Advances in prenatal screening for Down syndrome: II first trimester testing, integrated testing, and future directions.Clin Chim Acta. 2002 Oct;324(1-2):1-11. doi: 10.1016/s0009-8981(02)00187-0. Clin Chim Acta. 2002. PMID: 12204419 Review.
Cited by
-
Apolipoprotein A-I: insights from redox proteomics for its role in neurodegeneration.Proteomics Clin Appl. 2013 Jan;7(1-2):109-22. doi: 10.1002/prca.201200087. Proteomics Clin Appl. 2013. PMID: 23027708 Free PMC article. Review.
-
Comprehensive maternal serum proteomics identifies the cytoskeletal proteins as non-invasive biomarkers in prenatal diagnosis of congenital heart defects.Sci Rep. 2016 Jan 11;6:19248. doi: 10.1038/srep19248. Sci Rep. 2016. PMID: 26750556 Free PMC article.
-
Approaches for targeted proteomics and its potential applications in neuroscience.J Biosci. 2015 Sep;40(3):607-27. doi: 10.1007/s12038-015-9537-1. J Biosci. 2015. PMID: 26333406 Review.
-
Quantitative proteomic (iTRAQ) analysis of 1st trimester maternal plasma samples in pregnancies at risk for preeclampsia.J Biomed Biotechnol. 2012;2012:305964. doi: 10.1155/2012/305964. Epub 2012 Apr 10. J Biomed Biotechnol. 2012. PMID: 22570525 Free PMC article.
-
2D DIGE analysis of maternal plasma for potential biomarkers of Down Syndrome.Proteome Sci. 2011 Sep 19;9:56. doi: 10.1186/1477-5956-9-56. Proteome Sci. 2011. PMID: 21929753 Free PMC article.
References
-
- Eddleman KA, Malone FD, Sullivan L, et al. Pregnancy loss rates after midtrimester amniocentesis. Obstetrics and Gynecology. 2006;108(5):1067–1072. - PubMed
-
- Guetta E, Simchen MJ, Mammon-Daviko K, et al. Analysis of fetal blood cells in the maternal circulation: challenges, ongoing efforts, and potential solutions. Stem Cells and Development. 2004;13(1):93–99. - PubMed
-
- Lo YMD. Recent advances in fetal nucleic acids in maternal plasma. Journal of Histochemistry and Cytochemistry. 2005;53(3):293–296. - PubMed
-
- Wilson RD. Cell-free fetal DNA in the maternal circulation and its future uses in obstetrics. Journal of Obstetrics and Gynaecology Canada. 2005;27(1):54–62. - PubMed
-
- Spencer K. Aneuploidy screening in the first trimester. American Journal of Medical Genetics, Part C: Seminars in Medical Genetics. 2007;145(1):18–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

