Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer
- PMID: 19902470
- PMCID: PMC2935631
- DOI: 10.1002/pros.21078
Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer
Abstract
Objective: To determine if the levels of circulating myeloid-derived suppressor cells increase with progression of prostate cancer (PCa); to determine if such cells could contribute to the relative inefficiency of PCa immunotherapy.
Materials and methods: We analyzed peripheral blood mononuclear cells isolated from untreated PCa patients (uPCa; N = 18; mean age +/- SD: 72.1 +/- 6.9 years), tPCa (N = 22; 72.8 +/- 9.8 years) and age matched controls (AMC; N = 12; 68.8 +/- 7.5 years). We quantified surface marker phenotype, differentiation potential, effects on T cell proliferation and intracellular cytokines.
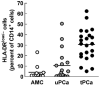
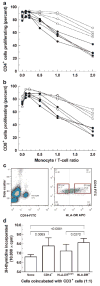
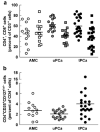
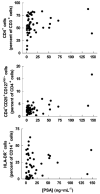
Results: We observed an unexpectedly high percentage of a type of myeloid-derived suppressor cells, CD14(+)HLA-DR(low/-) monocytes, in tPCa (30.7 +/- 15.0% of CD14(+) cells) relative to AMC (4.1 +/- 6.5%, P < 0.0001) and uPCa (10.6 +/- 14.3%, P = 0.0001). The levels of CD14(+) HLA-DR(low/-) cells were significantly correlated with circulating PSA levels and treatment with LHRH-agonist leuprolide in combination with either an antiandrogen or dexamethasone. Monocytes from tPCa inhibited autologous T cell proliferation statistically significantly more effectively than AMC monocytes and were defective in their ability to differentiate into phenotypically mature dendritic cells. Isolated CD14(+)HLA-DR(low/-) cells expressed higher levels of intracellular interleukin-10 and suppressed T cell proliferation more effectively than isolated CD14(+)HLA-DR(+) cells.
Conclusions: This is the first report of CD14(+) cells exhibiting reduced expression of HLA-DR molecules in PCa patients. These cells suppress immune cell function in vitro and, plausibly, in vivo, a finding that must be factored into the design of immunotherapy protocols for PCa patients.
Prostate 70: 443-455, 2010. (c) 2009 Wiley-Liss, Inc.
Conflict of interest statement
The authors have declared no financial conflict of interest in regards to this work.
Figures


Similar articles
-
Immunosuppressive CD14+HLA-DR(low)/- monocytes in B-cell non-Hodgkin lymphoma.Blood. 2011 Jan 20;117(3):872-81. doi: 10.1182/blood-2010-05-283820. Epub 2010 Nov 9. Blood. 2011. PMID: 21063024 Free PMC article.
-
Circulating immunosuppressive cells of prostate cancer patients before and after radical prostatectomy: profile comparison.Int J Urol. 2013 Oct;20(10):971-8. doi: 10.1111/iju.12086. Epub 2013 Feb 20. Int J Urol. 2013. PMID: 23421558
-
Activated human hepatic stellate cells induce myeloid derived suppressor cells from peripheral blood monocytes in a CD44-dependent fashion.J Hepatol. 2013 Sep;59(3):528-35. doi: 10.1016/j.jhep.2013.04.033. Epub 2013 May 9. J Hepatol. 2013. PMID: 23665041
-
The CD14+HLA-DRlo/neg Monocyte: An Immunosuppressive Phenotype That Restrains Responses to Cancer Immunotherapy.Front Immunol. 2019 May 22;10:1147. doi: 10.3389/fimmu.2019.01147. eCollection 2019. Front Immunol. 2019. PMID: 31191529 Free PMC article. Review.
-
Rebuilding immunity in cancer patients.Blood Cells Mol Dis. 2008 Jan-Feb;40(1):94-100. doi: 10.1016/j.bcmd.2007.06.025. Epub 2007 Sep 10. Blood Cells Mol Dis. 2008. PMID: 17827037 Free PMC article. Review.
Cited by
-
Effect of Early-Stage Human Breast Carcinoma on Monocyte Programming.Front Oncol. 2022 Feb 14;11:800235. doi: 10.3389/fonc.2021.800235. eCollection 2021. Front Oncol. 2022. PMID: 35237501 Free PMC article.
-
Sex-biased adaptive immune regulation in cancer development and therapy.iScience. 2022 Jul 4;25(8):104717. doi: 10.1016/j.isci.2022.104717. eCollection 2022 Aug 19. iScience. 2022. PMID: 35880048 Free PMC article. Review.
-
Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines.Cancer Immunol Immunother. 2012 Jun;61(6):827-38. doi: 10.1007/s00262-011-1143-y. Epub 2011 Nov 12. Cancer Immunol Immunother. 2012. PMID: 22080405 Free PMC article.
-
TransGEM: a molecule generation model based on Transformer with gene expression data.Bioinformatics. 2024 May 2;40(5):btae189. doi: 10.1093/bioinformatics/btae189. Bioinformatics. 2024. PMID: 38632084 Free PMC article.
-
The clinical and prognostic significance of CD14(+)HLA-DR(-/low) myeloid-derived suppressor cells in hepatocellular carcinoma patients receiving radiotherapy.Tumour Biol. 2016 Aug;37(8):10427-33. doi: 10.1007/s13277-016-4916-2. Epub 2016 Feb 5. Tumour Biol. 2016. PMID: 26846107
References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Thomas-Kaskel AK, Waller CF, Schultze-Seemann W, Veelken H. Immunotherapy with dendritic cells for prostate cancer. Int J Cancer. 2007;121:467–473. - PubMed
-
- Sciarra A, Lichtner M, Autran Gomez AM, Mastroianni C, Rossi R, Mengoni F, Cristini C, Gentilucci A, Vullo V, Di Silverio F. Characterization of circulating blood dendritic cell subsets DC123+ (lymphoid) and DC11c+ (myeloid) in prostate adenocarcinoma patients. Prostate. 2007;67:1–7. - PubMed
-
- Aalamian-Matheis M, Chatta GS, Shurin MR, Huland E, Huland H, Shurin GV. Inhibition of dendritic cell generation and function by serum from prostate cancer patients: Correlation with serum-free PSA. Adv Exp Med Biol. 2007;601:173–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

