Mouse organic solute transporter alpha deficiency enhances renal excretion of bile acids and attenuates cholestasis
- PMID: 19902485
- PMCID: PMC2819820
- DOI: 10.1002/hep.23265
Mouse organic solute transporter alpha deficiency enhances renal excretion of bile acids and attenuates cholestasis
Abstract
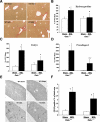
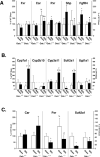
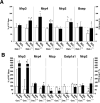
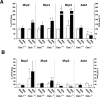
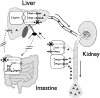
Organic solute transporter alpha-beta (Ostalpha-Ostbeta) is a heteromeric bile acid and sterol transporter that facilitates the enterohepatic and renal-hepatic circulation of bile acids. Hepatic expression of this basolateral membrane protein is increased in cholestasis, presumably to facilitate removal of toxic bile acids from the liver. In this study, we show that the cholestatic phenotype induced by common bile duct ligation (BDL) is reduced in mice genetically deficient in Ostalpha. Although Ostalpha(-/-) mice have a smaller bile acid pool size, which could explain lower serum and hepatic levels of bile acids after BDL, gallbladder bilirubin and urinary bile acid concentrations were significantly greater in Ostalpha(-/-) BDL mice, suggesting additional alternative adaptive responses. Livers of Ostalpha(-/-) mice had higher messenger RNA levels of constitutive androstane receptor (Car) than wild-type BDL mice and increased expression of Phase I enzymes (Cyp7a1, Cyp2b10, Cyp3a11), Phase II enzymes (Sult2a1, Ugt1a1), and Phase III transporters (Mrp2, Mrp3). Following BDL, the bile acid pool size increased in Ostalpha(-/-) mice and protein levels for the hepatic basolateral membrane export transporters, multidrug resistance-associated protein 3 (Mrp3) and Mrp4, and for the apical bilirubin transporter, Mrp2, were all increased. In the kidney of Ostalpha(-/-) mice after BDL, the apical bile acid uptake transporter Asbt is further reduced, whereas the apical export transporters Mrp2 and Mrp4 are increased, resulting in a significant increase in urinary bile acid excretion.
Conclusion: These findings indicate that loss of Ostalpha provides protection from liver injury in obstructive cholestasis through adaptive responses in both the kidney and liver that enhance clearance of bile acids into urine and through detoxification pathways most likely mediated by the nuclear receptor Car.
Figures

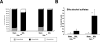
References
-
- Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, Madejczyl MS, et al. OSTα-OSTβ: A Major Basolateral Bile Acid and Steroid Transporter in Human Intestinal, Renal, and Biliary Epithelia. Hepatology. 2005;42:1270–1279. - PubMed
-
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metabolism. 2005;2:217–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
