Alternating pattern of stereochemistry in the nonactin macrocycle is required for antibacterial activity and efficient ion binding
- PMID: 19902940
- PMCID: PMC2879896
- DOI: 10.1021/ja9050235
Alternating pattern of stereochemistry in the nonactin macrocycle is required for antibacterial activity and efficient ion binding
Abstract
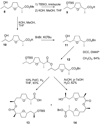
Nonactin is a polyketide antibiotic produced by Streptomyces griseus ETH A7796 and is an ionophore that is selective for K(+) ions. It is a cyclic tetraester generated from two monomers of (+)-nonactic acid and two of (-)-nonactic acid, arranged (+)-(-)-(+)-(-) so that nonactin has S4 symmetry and is achiral. To understand why achiral nonactin is the naturally generated diastereoisomer, we generated two alternate diastereoisomers of nonactin, one prepared solely from (+)-nonactic acid and one prepared solely from (-)-nonactic acid, referred to here as 'all-(+)-nonactin' and 'all-(-)-nonactin', respectively. Both non-natural diastereoisomers were 500-fold less active against gram positive organisms than nonactin confirming that the natural stereochemistry is necessary for biological activity. We used isothermal calorimetry to obtain the K(a), DeltaG, DeltaH, and DeltaS of formation for the K(+), Na(+), and NH(4)(+) complexes of nonactin and all-(-)-nonactin; the natural diastereoisomer bound K(+) 880-fold better than all-(-)-nonactin. A picrate partitioning assay confirmed that all-(-)-nonactin, unlike nonactin, could not partition K(+) ions into organic solvent. To complement the thermodynamic data we used a simple model system to show that K(+) transport was facilitated by nonactin but not by all-(-)-nonactin. Modeling of the K(+) complexes of nonactin and all-(-)-nonactin suggested that poor steric interactions in the latter complex precluded tight binding to K(+). Overall, the data show that both enantiomers of nonactic acid are needed for the formation of a nonactin diastereoisomer that can act as an ionophore and has antibacterial activity.
Figures




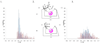

Similar articles
-
Nonactin biosynthesis: setting limits on what can be achieved with precursor-directed biosynthesis.Bioorg Med Chem Lett. 2009 Feb 15;19(4):1233-5. doi: 10.1016/j.bmcl.2008.12.096. Epub 2008 Dec 30. Bioorg Med Chem Lett. 2009. PMID: 19167217 Free PMC article.
-
Nonactin biosynthesis: the initial committed step is the condensation of acetate (malonate) and succinate.J Am Chem Soc. 2002 Mar 27;124(12):2894-902. doi: 10.1021/ja016965f. J Am Chem Soc. 2002. PMID: 11902879
-
Nonactin biosynthesis: the product of nonS catalyzes the formation of the furan ring of nonactic acid.Antimicrob Agents Chemother. 1999 Jul;43(7):1662-8. doi: 10.1128/AAC.43.7.1662. Antimicrob Agents Chemother. 1999. PMID: 10390219 Free PMC article.
-
Structures, Synthesis and Biological Activities of Nonactic Acid and Its Derivatives.Curr Med Chem. 2021;28(42):8673-8691. doi: 10.2174/0929867328666210628144347. Curr Med Chem. 2021. PMID: 34182902 Review.
-
Advances in macrocyclic peptide-based antibiotics.Bioorg Med Chem. 2018 Jun 1;26(10):2850-2858. doi: 10.1016/j.bmc.2017.08.006. Epub 2017 Aug 19. Bioorg Med Chem. 2018. PMID: 28886999 Review.
Cited by
-
Functionalization of silk with actinomycins from Streptomyces anulatus BV365 for biomedical applications.Front Bioeng Biotechnol. 2024 Sep 19;12:1466757. doi: 10.3389/fbioe.2024.1466757. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39364265 Free PMC article.
-
Inhibitory Effects of Macrotetrolides from Streptomyces spp. On Zoosporogenesis and Motility of Peronosporomycete Zoospores Are Likely Linked with Enhanced ATPase Activity in Mitochondria.Front Microbiol. 2016 Nov 18;7:1824. doi: 10.3389/fmicb.2016.01824. eCollection 2016. Front Microbiol. 2016. PMID: 27917156 Free PMC article.
-
Diversity, Bioactivity Profiling and Untargeted Metabolomics of the Cultivable Gut Microbiota of Ciona intestinalis.Mar Drugs. 2020 Dec 24;19(1):6. doi: 10.3390/md19010006. Mar Drugs. 2020. PMID: 33374243 Free PMC article.
-
BtsT, a Novel and Specific Pyruvate/H+ Symporter in Escherichia coli.J Bacteriol. 2017 Dec 20;200(2):e00599-17. doi: 10.1128/JB.00599-17. Print 2018 Jan 15. J Bacteriol. 2017. PMID: 29061664 Free PMC article.
-
A novel calix[4]pyrrole derivative as a potential anticancer agent that forms genotoxic adducts with DNA.Sci Rep. 2018 Jul 23;8(1):11075. doi: 10.1038/s41598-018-29314-9. Sci Rep. 2018. PMID: 30038406 Free PMC article.
References
-
- Corbaz R, Ettinger L, Gaumann E, Keller-Schlierlein W, Kradolfer F, Kyburz E, Neipp L, Prelog V, Zahner H. Helv. Chim. Acta. 1955;38:1445.
-
- Meyers E, Pansy FE, Perlman D, Smith DA, Weisenborn FL. J. Antibiot. 1965;18:128. - PubMed
-
- Gerlach H, Hutter R, Keller-Schlierlein W, Seibl J, Zahner H. Helv. Chim. Acta. 1967;50:1782–1793. - PubMed
-
- Keller-Schierlein W, Gerlach H. Fortschritte d. Chem. Org. Naturstoffe. 1968;26:161–189. - PubMed
-
- Borrel MN, Pereira E, Fiallo M, Garnier-Suillerot A. Eur. J. Biochem. 1994;223:125–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

