Palladium-catalyzed enantioselective addition of two distinct nucleophiles across alkenes capable of quinone methide formation
- PMID: 19902942
- PMCID: PMC2792916
- DOI: 10.1021/ja909030c
Palladium-catalyzed enantioselective addition of two distinct nucleophiles across alkenes capable of quinone methide formation
Abstract
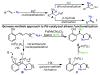
A sequential intramolecular-intermolecular enantioselective alkene difunctionalization reaction has been developed which is thought to proceed through Pd-catalyzed quinone methide formation. The synthesis of new chiral heterocyclic compounds with adjacent chiral centers is achieved in enantiomeric ratios up to 99:1 and diastereomeric ratios up to 10:1.
Figures
References
-
- Kolb HC, VanNieuwenhze MS, Sharpless KB. Chem Rev. 1994;94:2483.
-
-
For recent examples, see: Zabawa TP, Kasi D, Chemler SR. J Am Chem Soc. 2005;127:11250.Zeng W, Chemler SR. J Am Chem Soc. 2007;129:12948.Fuller PH, Chemler SR. Org Lett. 2007;9:5477.Michaelis DJ, Shaffer CJ, Yoon TP. J Am Chem Soc. 2007;129:1866.Fuller PH, Kim JW, Chemler SR. J Am Chem Soc. 2008;130:17638.Michaelis DJ, Ischay MA, Yoon TP. J Am Chem Soc. 2008;130:6610.Paderes MC, Chemler SR. Org Lett. 2009;11:1915.Donohoe TJ, Callens CKA, Thompson AL. Org Lett. 2009;11:2305.Benkovics T, Du J, Guzei IA, Yoon TP. J Org Chem. 2009;74:5545.For a review of alkene 1,2-diamination see: Cardona F, Goti A. Nature Chem. 2009;1:269.
-
-
-
For a review of pioneering work in this area, see: Bäckvall J-E. Metal-Catalyzed Cross-Coupling Reactions. 2. Vol. 2. 2004. p. 479.Bäckvall JE, Nordberg RE. J Am Chem Soc. 1981;103:4959.For a leading reference on alkoxycarbonylation, see: Semmelhack MF, Bodurow C. J Am Chem Soc. 1984;106:1496.For a review of advances in Pd-catalyzed alkene difunctionalization, see: Jensen KH, Sigman MS. Org Biomol Chem. 2008;6:4083.Kalyani D, Sanford MS. J Am Chem Soc. 2008;130:2150.Wang A, Jiang H, Chen H. J Am Chem Soc. 2009;131:3846.Rosewall CF, Sibbald PA, Liskin DV, Michael FE. J Am Chem Soc. 2009;131:9488.Rodriguez A, Moran WJ. Eur J Org Chem. 2009:1313.Urkalan KB, Sigman MS. Angew Chem, Int Ed. 2009;48:3146.Tsujihara T, Takenaka K, Onitsuka K, Hatanaka M, Sasai H. J Am Chem Soc. 2009;131:3452.
-
-
- Beccalli EM, Broggini G, Martinelli M, Sottocornola S. Chem Rev. 2007;107:5318. - PubMed
-
-
Schultz MJ, Sigman MS. J Am Chem Soc. 2006;128:1460.Zhang Y, Sigman MS. J Am Chem Soc. 2007;129:3076.For other examples of Pd-catalyzed dioxygenation of o-vinyl phenols, see: Chevrin C, Le Bras J, Henin F, Muzart J. Synthesis. 2005:2615.Thiery E, Chevrin C, Le Bras J, Harakat D, Muzart J. J Org Chem. 2007;72:1859.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources