Optimization and validation of a robust human T-cell culture method for monitoring phenotypic and polyfunctional antigen-specific CD4 and CD8 T-cell responses
- PMID: 19903103
- PMCID: PMC2932850
- DOI: 10.3109/14653240903136987
Optimization and validation of a robust human T-cell culture method for monitoring phenotypic and polyfunctional antigen-specific CD4 and CD8 T-cell responses
Abstract
Background aims: Monitoring cellular immune responses is one prerequisite for the rational development of cancer vaccines.
Methods: We describe an extensive effort to optimize and validate quantitatively an in vitro T-cell culture method by determining the phenotype and function of both CD4(+) and CD8(+) T cells, including measurement of the phenotype markers CCR7, CD45RA, CD28 and CD27 and the functional markers interferon (IFN)-gamma, interleukin (IL)-2, macrophage inflammatory protein (MIP)-1beta, tumor necrosis factor (TNF)-alpha and CD107a.
Results: Autologous peripheral blood mononuclear cells (PBMC) were potent stimulators that expanded antigen (Ag)-specific CD8(+) T cells during short-term culture with the addition of IL-2 and IL-15 cytokines. Polyfunctional Ag-specific CD4(+) and CD8(+) T cells were detectable using this method.
Conclusions: Our culture system represents a robust human T-cell culture protocol that permits phenotypic, quantitative and qualitative evaluation of vaccine-induced CD4(+) and CD8(+) T-cell responses.
Figures

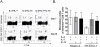
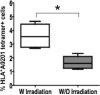
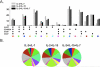


Comment in
-
Optimizing T-cell expansion: have we reached the limit?Cytotherapy. 2009;11(7):813-4. doi: 10.3109/14653240903387163. Cytotherapy. 2009. PMID: 19903094 No abstract available.
References
-
- Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. - PubMed
-
- Gnjatic S, Nishikawa H, Jungbluth AA, Gure AO, Ritter G, Jager E, Knuth A, Chen YT, Old LJ. NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. - PubMed
-
- Romero P, Valmori D, Pittet MJ, Zippelius A, Rimoldi D, Levy F, Dutoit V, Ayyoub M, Rubio-Godoy V, Michielin O, et al. Antigenicity and immunogenicity of Melan-A/MART-1 derived peptides as targets for tumor reactive CTL in human melanoma. Immunol Rev. 2002;188:81–96. - PubMed
-
- Janetzki S, Panageas KS, Ben-Porat L, Boyer J, Britten CM, Clay TM, Kalos M, Maecker HT, Romero P, Yuan J, et al. Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI). Cancer Immunol Immunother. 2008;57:303–315. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

