Prednisolone Trial: Study protocol for a randomised controlled trial of prednisolone for women with idiopathic recurrent miscarriage and raised levels of uterine natural killer (uNK) cells in the endometrium
- PMID: 19903335
- PMCID: PMC2785777
- DOI: 10.1186/1745-6215-10-102
Prednisolone Trial: Study protocol for a randomised controlled trial of prednisolone for women with idiopathic recurrent miscarriage and raised levels of uterine natural killer (uNK) cells in the endometrium
Abstract
Background: Idiopathic recurrent miscarriage is defined as 3 consecutive pregnancy losses with no contributing features found on investigations. At present there are no treatments of proven efficacy for idiopathic recurrent miscarriage. Uterine natural killer (uNK) cells, the most predominant leucocyte in the endometrium are adjacent to foetal trophoblast cells and thought to be involved in implantation. The exact mechanisms of how uNK cells affect implantation are not clear but are probably through the regulation of angiogenesis. Multiple studies have shown an association between high density of uterine natural killer cells and recurrent miscarriage. We have shown that prednisolone reduces the number of uNK cells in the endometrium. The question remains as to whether reducing the number of uNK cells improves pregnancy outcome.
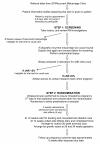
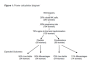
Methods: We propose a randomised, double-blind, placebo controlled trial of prednisolone with a pilot phase to assess feasibility of recruitment, integrity of trial procedures, and to generate data to base future power calculations. The primary aim is to investigate whether prednisolone therapy during the first trimester of pregnancy is able to improve live birth rates in patients with idiopathic recurrent miscarriage and raised uNK cells in the endometrium. Secondary outcomes include conception rate, karyotype of miscarriage, miscarriages (first and second trimester), stillbirths, pregnancy complications, gestational age at delivery, congenital abnormality and side effects of steroids. The trial has 2 stages: i) screening of non-pregnant women and ii) randomisation of the pregnant cohort. All patients who fit the inclusion criteria (<40 years old, > or =3 consecutive miscarriages with no cause found and no contraindications to prednisolone therapy) will be asked to consent to an endometrial biopsy in the mid-luteal phase to assess their levels of uNK cells. Women with high levels of uNK cells (> or =5%), will be randomised to either prednisolone or placebo when a pregnancy is confirmed. Follow-up includes 2 weekly ultrasound scans in the first trimester, an anomaly scan at 20 weeks gestation, growth scans at 28 and 34 weeks gestation and a postnatal follow-up at 6 weeks.
Trial registration: Current Controlled Trials ISRCTN28090716.
Figures
References
-
- Quenby SM, Farquharson RG. Predicting recurring miscarriage: What is important? Obstet Gynaecol. 1993;82:132–138. - PubMed
-
- Royal College of Obstetricians and Gynaecologists. Guideline No. 17. London: RCOG; 2003. The investigation and treatment of couples with recurrent miscarriage.
-
- Porter TF, LaCoursiere Y, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. 2006;2:CD000112. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources