Chapter 9 - Nanoliposomal dry powder formulations
- PMID: 19903555
- PMCID: PMC3766362
- DOI: 10.1016/S0076-6879(09)64009-X
Chapter 9 - Nanoliposomal dry powder formulations
Abstract
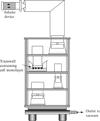
Liposomal dry powder formulations (DPFs) have proven their superiority over conventional DPFs due to favorably improved pharmacokinetics and pharmacodynamics of entrapped drugs, and thus, reduced local and systemic toxicities. Nanoliposomal DPFs (NLDPFs) provide stable, high aerosolization efficiency to deep lung, prolonged drug release, slow systemic dilution, and avoid macrophage uptake of encapsulated drug by carrier-based delivery of nano-range liposomes. This chapter describes methods of preparation of nanoliposomes (NLs) and NLDPFs, using various techniques, and their characterization with respect to size distribution, flow behavior, in vitro drug release profile, lung deposition, cellular uptake and cytotoxicity, and in vivo pharmacokinetics and pharmacodynamics. Some examples have been detailed for better understanding of the methods of preparation and evaluation of NLDPFs by investigators.
Figures






Similar articles
-
Recent advances in liposomal dry powder formulations: preparation and evaluation.Expert Opin Drug Deliv. 2009 Jan;6(1):71-89. doi: 10.1517/17425240802652309. Expert Opin Drug Deliv. 2009. PMID: 19236209 Review.
-
Nano-liposomal dry powder inhaler of tacrolimus: preparation, characterization, and pulmonary pharmacokinetics.Int J Nanomedicine. 2007;2(4):675-88. Int J Nanomedicine. 2007. PMID: 18203434 Free PMC article.
-
Nano-liposomal dry powder inhaler of Amiloride Hydrochloride.J Nanosci Nanotechnol. 2006 Sep-Oct;6(9-10):3001-9. doi: 10.1166/jnn.2006.405. J Nanosci Nanotechnol. 2006. PMID: 17048511
-
Development and evaluation of a dry powder formulation of liposome-encapsulated oseltamivir phosphate for inhalation.Drug Deliv. 2015;22(5):608-18. doi: 10.3109/10717544.2013.863526. Epub 2013 Dec 4. Drug Deliv. 2015. PMID: 24299495
-
Stabilization of liposomes during drying.Expert Opin Drug Deliv. 2011 Mar;8(3):375-88. doi: 10.1517/17425247.2011.553219. Epub 2011 Feb 4. Expert Opin Drug Deliv. 2011. PMID: 21294603 Review.
Cited by
-
Development of Optimized, Inhalable, Gemcitabine-Loaded Gelatin Nanocarriers for Lung Cancer.J Aerosol Med Pulm Drug Deliv. 2017 Oct;30(5):299-321. doi: 10.1089/jamp.2015.1286. Epub 2017 Mar 9. J Aerosol Med Pulm Drug Deliv. 2017. PMID: 28277892 Free PMC article.
-
Formulation development of itraconazole PEGylated nano-lipid carriers for pulmonary aspergillosis using hot-melt extrusion technology.Int J Pharm X. 2021 Mar 3;3:100074. doi: 10.1016/j.ijpx.2021.100074. eCollection 2021 Dec. Int J Pharm X. 2021. PMID: 33748741 Free PMC article.
-
Aerosol Delivery of siRNA to the Lungs. Part 1: Rationale for Gene Delivery Systems.Kona. 2016 Feb 28;33:63-85. doi: 10.14356/kona.2016014. Epub 2015 Sep 30. Kona. 2016. PMID: 27081214 Free PMC article.
-
Biphasic burst and sustained transdermal delivery in vivo using an AI-optimized 3D-printed MN patch.Int J Pharm. 2023 Apr 5;636:122647. doi: 10.1016/j.ijpharm.2023.122647. Epub 2023 Feb 6. Int J Pharm. 2023. PMID: 36754185 Free PMC article.
-
Formulation of Topical Flurbiprofen Solid Lipid Nanoparticle Gel Formulation Using Hot Melt Extrusion Technique.AAPS PharmSciTech. 2022 Sep 16;23(7):257. doi: 10.1208/s12249-022-02410-w. AAPS PharmSciTech. 2022. PMID: 36114430 Free PMC article.
References
-
- Aerosols, Nasal Sprays, Metered Dose Inhalers, and Dry Powder Inhalers. USP 30 NF. 2007;25:601–607.
-
- Akwete LA, Hsu L. Leuprolide and other LH-RH analogues in stability and characterization of protein and peptide drugs: Case histories. In: Wang YJ, Pearlman R, editors. “Formation, Characterization, and Stability of Protein Drugs,”. New York: Plenum Press; 1993. pp. 159–199.
-
- Anabousi S, Bakowsky U, Schneider M, Huwer H, Lehr CM, Ehrhardt C. In vitro assessment of transferrin-conjugated liposomes as drug delivery systems for inhalation therapy of lung cancer. Eur. J. Pharm. Sci. 2006;29:367–374. - PubMed
-
- Application Note by Malvern Instruments. Size and zeta potential characterization of anionic and cationic liposomes on the Zetasizer Nano
-
- Ashurst II, Malton A, Prime D, Sumby B. Latest advances in the development of dry powder inhalers. Pharm. Sci. Technol. 2000;3:246–256. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

