Class II-associated invariant chain peptide down-modulation enhances the immunogenicity of myeloid leukemic blasts resulting in increased CD4+ T-cell responses
- PMID: 19903675
- PMCID: PMC2833080
- DOI: 10.3324/haematol.2009.010595
Class II-associated invariant chain peptide down-modulation enhances the immunogenicity of myeloid leukemic blasts resulting in increased CD4+ T-cell responses
Abstract
Background: Disease recurrence in patients with acute myeloid leukemia may be partially explained by the escape of leukemic blasts from CD4(+) T-cell recognition. The current study investigates the role of aberrant HLA class II antigen presentation on leukemic blasts by determining both the clinical and functional impact of the class II-associated invariant chain peptide (CLIP).
Design and methods: The levels of expression of CLIP and HLA-DR on blood and bone marrow samples from 207 patients with acute myeloid leukemia were correlated with clinical outcome. Irradiated CLIP(-) and CLIP(+) leukemic blasts were compared for their ability to induce CD4(+) T cells during mixed leukocyte reactions. To discriminate between these blasts, we down-modulated CLIP expression on myeloid leukemic cell lines by RNA interference of the invariant chain, a chaperone protein critically involved in HLA-DR processing, and performed flow cytometric sorting for their isolation from primary acute myeloid leukemia samples.
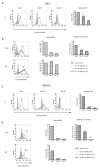
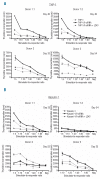
Results: We found that patients with leukemic blasts characterized by a high amount of HLA-DR occupied by CLIP (relative amount of CLIP) had a significantly shortened disease-free survival. The clear reductions in amount of HLA-DR occupied by CLIP on blasts of the THP-1 and Kasumi-1 myeloid leukemic cell lines after treatment with invariant chain short interfering RNA resulted in enhanced rates of allogeneic CD4(+) T-cell proliferation. Similar findings were obtained in an autologous setting, in which there were strong increases in proliferation of remission CD4(+) T cells stimulated with CLIP(-)-sorted leukemic blasts from HLA-DR(+) acute myeloid leukemia patients, in contrast to CLIP(+)-sorted leukemic blasts from the same patients.
Conclusions: These data highlight the relevance of CLIP expression on leukemic blasts and the potential of CLIP as a target for immunomodulatory strategies to enhance HLA class II antigen presentation and CD4(+) T-cell reactivity in acute myeloid leukemia.
Figures


References
-
- Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341(14):1051–62. - PubMed
-
- Schreiber RD. Cancer vaccines 2004 opening address: the molecular and cellular basis of cancer immunosurveillance and immunoediting. Cancer Immun. 2005;5 (Suppl 1):1. - PubMed
-
- Greiner J, Schmitt M, Li L, Giannopoulos K, Bosch K, Schmitt A, et al. Expression of tumor-associated antigens in acute myeloid leukemia: implications for specific immunotherapeutic approaches. Blood. 2006;108 (13):4109–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

