Effect of granulocyte/macrophage colony-stimulating factor on vaccination with an allogeneic whole-cell melanoma vaccine
- PMID: 19903777
- PMCID: PMC2920049
- DOI: 10.1158/1078-0432.CCR-09-1540
Effect of granulocyte/macrophage colony-stimulating factor on vaccination with an allogeneic whole-cell melanoma vaccine
Abstract
Purpose: The availability of a variety of immune response modifiers creates an opportunity for improved efficacy of immunotherapy, but it also leads to uncertainty in how to combine agents and how to assess those combinations. We sought to assess the effect of the addition of granulocyte/macrophage colony-stimulating factor (GM-CSF) to vaccination with a melanoma vaccine.
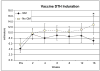
Experimental design: Ninety-seven patients with resected melanoma (stage II-IV) were enrolled, stratified by stage, and randomized to receive a cellular melanoma vaccine with or without GM-CSF. The primary endpoint was delayed-type hypersensitivity (DTH) response to melanoma cells. Antibody responses, peripheral leukocyte counts, and survival were also examined.
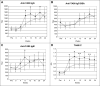
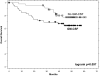
Results: The GM-CSF arm showed enhanced antibody responses with an increase in IgM titer against the TA90 antigen and increased TA90 immune complexes. This arm also had diminished antimelanoma cell delayed-type hypersensitivity response. Peripheral blood leukocyte profiles showed increases in eosinophils and basophils with decreased monocytes in the GM-CSF arm. These immune changes were accompanied by an increase in early melanoma deaths and a trend toward worse survival with GM-CSF.
Conclusion: These data suggest that GM-CSF is not helpful as an immune adjuvant in this dose and schedule and raise concern that it may be harmful. Based on the discordant findings of an immune endpoint and clinical outcome, the use of such surrogate endpoints in selecting treatments for further evaluation must be done with a great deal of caution.
Figures

References
-
- Mach N, Gillessen S, Wilson SB, et al. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–46. - PubMed
-
- Fischer HG, Frosch S, Reske K, et al. Granulocyte-macrophage colony-stimulating factor activates macrophages derived from bone marrow cultures to synthesis of MHC class II molecules and to augmented antigen presentation function. J Immunol. 1988;141:3882–8. - PubMed
-
- Spitler LE, Grossbard ML, Ernstoff MS, et al. Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2000;18:1614–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

