Detecting an overall survival benefit that is derived from progression-free survival
- PMID: 19903805
- PMCID: PMC4137232
- DOI: 10.1093/jnci/djp369
Detecting an overall survival benefit that is derived from progression-free survival
Abstract
Background: Whether progression-free survival (PFS) or overall survival (OS) is the more appropriate endpoint in clinical trials of metastatic cancer is controversial. In some disease and treatment settings, an improvement in PFS does not result in an improved OS.
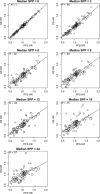
Methods: We partitioned OS into two parts and expressed it as the sum of PFS and survival postprogression (SPP). We simulated randomized clinical trials with two arms that had respective medians for PFS of 6 and 9 months. We assumed no treatment difference in median SPP. We found the probability of a statistically significant benefit in OS for various median SPP and observed P values for PFS. We compared the sample sizes required for PFS vs OS for various median SPP. We compare our results with the literature regarding surrogacy of PFS for OS by use of the correlation between hazard ratios for PFS and OS. All statistical tests were two-sided.
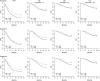
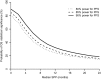
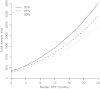
Results: For a trial with observed P value for improvement in PFS of .001, there was a greater than 90% probability for statistical significance in OS if median SPP was 2 months but less than 20% if median SPP was 24 months. For a trial requiring 280 patients to detect a 3-month difference in PFS, 350 and 2440 patients, respectively, were required to have the same power for detecting a real difference in OS that is carried over from the 3-month benefit in PFS when the median SPP was 2 and 24 months.
Conclusions: Addressing SPP is important in understanding treatment effects. For clinical trials with a PFS benefit, lack of statistical significance in OS does not imply lack of improvement in OS, especially for diseases with long median SPP. Although there may be no treatment effect on SPP, its variability so dilutes the OS comparison that statistical significance is likely lost. OS is a reasonable primary endpoint when median SPP is short but is too high a bar when median SPP is long, such as longer than 12 months.
Figures
References
-
- Albain KS. Discussion presented at 2008 American Society of Clinical Oncology Annual Meeting. Chicago, IL: June 2008. Adding a new agent to the old in metastatic breast cancer: progress, promise, and challenges. http://www.asco.org//ASCO/Abstracts+%26+Virtual+Meeting/Virtual+Meeting?.... Accessed April 3, 2009.
-
- Mayfield E. Progression-free survival: patient benefit or lower standard. Life Raft Group. 2007 http://www.liferaftgroup.org/news_sci_articles/pfs_benefit.html. Accessed March 23, 2009.
-
- Allison M. Trouble at the office [abstract 23] Nat Biotechnol. 2008;26(9):967–969. - PubMed
-
- Chakravarty A, Sridhara R. Use of progression-free survival as a surrogate marker in oncology trials: some regulatory issues. Stat Methods Med Res. 2008;17(5):515–518. - PubMed
-
- Panageas KS, Ben-Porat L, Dickler MN, Chapman PB, Schrag D. When you look matters: the effect of assessment schedule on progression-free survival. J Natl Cancer Inst. 2007;99(6):428–432. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

