N terminus is key to the dominant negative suppression of Ca(V)2 calcium channels: implications for episodic ataxia type 2
- PMID: 19903821
- PMCID: PMC2801285
- DOI: 10.1074/jbc.M109.065045
N terminus is key to the dominant negative suppression of Ca(V)2 calcium channels: implications for episodic ataxia type 2
Abstract
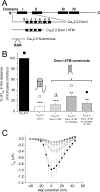
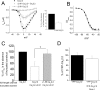
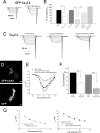
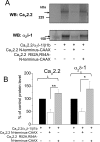
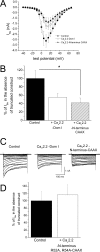
Expression of the calcium channels Ca(V)2.1 and Ca(V)2.2 is markedly suppressed by co-expression with truncated constructs containing Domain I. This is the basis for the phenomenon of dominant negative suppression observed for many of the episodic ataxia type 2 mutations in Ca(V)2.1 that predict truncated channels. The process of dominant negative suppression has been shown previously to stem from interaction between the full-length and truncated channels and to result in downstream consequences of the unfolded protein response and endoplasmic reticulum-associated protein degradation. We have now identified the specific domain that triggers this effect. For both Ca(V)2.1 and Ca(V)2.2, the minimum construct producing suppression was the cytoplasmic N terminus. Suppression was enhanced by tethering the N terminus to the membrane with a CAAX motif. The 11-amino acid motif (including Arg(52) and Arg(54)) within the N terminus, which we have previously shown to be required for G protein modulation, is also essential for dominant negative suppression. Suppression is prevented by addition of an N-terminal tag (XFP) to the full-length and truncated constructs. We further show that suppression of Ca(V)2.2 currents by the N terminus-CAAX construct is accompanied by a reduction in Ca(V)2.2 protein level, and this is also prevented by mutation of Arg(52) and Arg(54) to Ala in the truncated construct. Taken together, our evidence indicates that both the extreme N terminus and the Arg(52), Arg(54) motif are involved in the processes underlying dominant negative suppression.
Figures


Similar articles
-
Dominant-negative synthesis suppression of voltage-gated calcium channel Cav2.2 induced by truncated constructs.J Neurosci. 2001 Nov 1;21(21):8495-504. doi: 10.1523/JNEUROSCI.21-21-08495.2001. J Neurosci. 2001. PMID: 11606638 Free PMC article.
-
Dominant-negative calcium channel suppression by truncated constructs involves a kinase implicated in the unfolded protein response.J Neurosci. 2004 Jun 9;24(23):5400-9. doi: 10.1523/JNEUROSCI.0553-04.2004. J Neurosci. 2004. PMID: 15190113 Free PMC article.
-
A CaV2.1 N-terminal fragment relieves the dominant-negative inhibition by an Episodic ataxia 2 mutant.Neurobiol Dis. 2016 Sep;93:243-56. doi: 10.1016/j.nbd.2016.05.020. Epub 2016 May 31. Neurobiol Dis. 2016. PMID: 27260834 Free PMC article.
-
Beta-subunits promote the expression of Ca(V)2.2 channels by reducing their proteasomal degradation.J Biol Chem. 2011 Mar 18;286(11):9598-611. doi: 10.1074/jbc.M110.195909. Epub 2011 Jan 13. J Biol Chem. 2011. PMID: 21233207 Free PMC article.
-
Site-specific regulation of CA(V)2.2 channels by protein kinase C isozymes betaII and epsilon.Neuroscience. 2009 Mar 17;159(2):618-28. doi: 10.1016/j.neuroscience.2008.12.047. Epub 2009 Jan 3. Neuroscience. 2009. PMID: 19167461
Cited by
-
Inhibition of synaptic transmission and G protein modulation by synthetic CaV2.2 Ca²+ channel peptides.J Physiol. 2011 Jul 1;589(Pt 13):3085-101. doi: 10.1113/jphysiol.2010.204735. Epub 2011 Apr 26. J Physiol. 2011. PMID: 21521766 Free PMC article.
-
The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential.Pharmacol Rev. 2015 Oct;67(4):821-70. doi: 10.1124/pr.114.009654. Pharmacol Rev. 2015. PMID: 26362469 Free PMC article. Review.
-
The life cycle of voltage-gated Ca2+ channels in neurons: an update on the trafficking of neuronal calcium channels.Neuronal Signal. 2021 Feb 23;5(1):NS20200095. doi: 10.1042/NS20200095. eCollection 2021 Apr. Neuronal Signal. 2021. PMID: 33664982 Free PMC article. Review.
-
Regulation of Ca(V)2 calcium channels by G protein coupled receptors.Biochim Biophys Acta. 2013 Jul;1828(7):1629-43. doi: 10.1016/j.bbamem.2012.10.004. Epub 2012 Oct 12. Biochim Biophys Acta. 2013. PMID: 23063655 Free PMC article. Review.
-
A naturally occurring truncated Cav1.2 α1-subunit inhibits Ca2+ current in A7r5 cells.Am J Physiol Cell Physiol. 2013 Oct 15;305(8):C896-905. doi: 10.1152/ajpcell.00217.2013. Epub 2013 Aug 7. Am J Physiol Cell Physiol. 2013. PMID: 23926129 Free PMC article.
References
-
- Catterall W. A. (2000) Annu. Rev. Cell Dev. Biol. 16, 521–555 - PubMed
-
- Ertel E. A., Campbell K. P., Harpold M. M., Hofmann F., Mori Y., Perez-Reyes E., Schwartz A., Snutch T. P., Tanabe T., Birnbaumer L., Tsien R. W., Catterall W. A. (2000) Neuron 25, 533–535 - PubMed
-
- Pietrobon D. (2002) Mol. Neurobiol. 25, 31–50 - PubMed
-
- Ophoff R. A., Terwindt G. M., Vergouwe M. N., van Eijk R., Oefner P. J., Hoffman S. M., Lamerdin J. E., Mohrenweiser H. W., Bulman D. E., Ferrari M., Haan J., Lindhout D., van Ommen G. J., Hofker M. H., Ferrari M. D., Frants R. R. (1996) Cell 87, 543–552 - PubMed
-
- Denier C., Ducros A., Vahedi K., Joutel A., Thierry P., Ritz A., Castelnovo G., Deonna T., Gérard P., Devoize J. L., Gayou A., Perrouty B., Soisson T., Autret A., Warter J. M., Vighetto A., Van Bogaert P., Alamowitch S., Roullet E., Tournier-Lasserve E. (1999) Neurology 52, 1816–1821 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

